当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Identifying Activity Trends for the Electrochemical Production of H2O2 on M–N–C Single-Atom Catalysts Using Theoretical Kinetic Computations
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2022-06-15 , DOI: 10.1021/acs.jpcc.2c02803 Jianhua Shen 1 , Yingqiang Wen 1 , Haibo Jiang 1 , Shengwei Yu 1 , Chunxiao Dong 1 , Yu Fan 2 , Bin Liu 3 , Chunzhong Li 2
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2022-06-15 , DOI: 10.1021/acs.jpcc.2c02803 Jianhua Shen 1 , Yingqiang Wen 1 , Haibo Jiang 1 , Shengwei Yu 1 , Chunxiao Dong 1 , Yu Fan 2 , Bin Liu 3 , Chunzhong Li 2
Affiliation
|
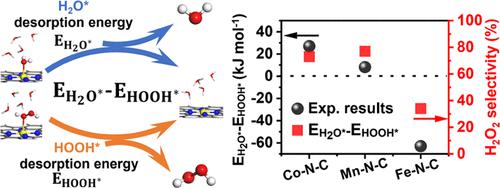
Metal–nitrogen–carbon (M–N–C) single-atom catalysts (SACs) have shown high potential to generate H2O2 through the two-electron oxygen reduction reaction (2e– ORR) pathway in an acidic electrolyte. However, there is still a lack of effective kinetic computation to reveal M–N–C SACs’ intrinsic catalytical performance toward 2e– ORR. Here, we combine the experimental and computational efforts to study the 2e– ORR activity trends of M–N–C SACs (M = Co, Fe, and Mn). The experimental results show that Co–N–C and Mn–N–C SACs favor the 2e– ORR pathway but Fe–N–C SAC favors the 4e– ORR pathway. Using systematic kinetic calculation, we demonstrated that desorption of HOOH* or H2O* was a real determining step of the ORR pathway on M–N–C SACs. The difference in the desorption energy between H2O* and HOOH* reaction oxygen intermediates (EH2O*– EHOOH*) on a metal active center is a credible descriptor to illustrate and predict the H2O2 selectivity of M–N–C SACs. The predicted high H2O2 selectivity of Ni–N–C SACs from the descriptor is consistent with the experimental results. This study clarifies the mechanism of ORR on M–N–C SACs and identifies the determining factors of the ORR pathway, providing new insights into the rational design of high-H2O2-selectivity M–N–C SACs.
中文翻译:

使用理论动力学计算确定 M–N–C 单原子催化剂上电化学生产 H2O2 的活性趋势
金属-氮-碳 (M-N-C) 单原子催化剂 (SAC) 已显示出在酸性电解质中通过双电子氧还原反应 (2e - ORR) 途径产生 H 2 O 2的高潜力。然而,仍然缺乏有效的动力学计算来揭示 M-N-C SACs 对 2e - ORR 的内在催化性能。在这里,我们结合实验和计算工作来研究M-N-C SAC(M = Co、Fe 和 Mn)的 2e - ORR 活性趋势。实验结果表明,Co-N-C 和 Mn-N-C SAC 有利于 2e - ORR 途径,但 Fe-N-C SAC 有利于 4e -ORR 通路。使用系统动力学计算,我们证明 HOOH* 或 H 2 O* 的解吸是 M-N-C SAC 上 ORR 途径的真正决定性步骤。H 2 O* 和 HOOH* 反应氧中间体 ( E H 2 O* – E HOOH* ) 在金属活性中心上的解吸能差异是说明和预测 M– 的 H 2 O 2选择性的可靠描述符N–C SAC。预测的高 H 2 O 2描述符中 Ni-N-C SAC 的选择性与实验结果一致。本研究阐明了 ORR 对 M-N-C SACs 的作用机制,并确定了 ORR 途径的决定因素,为合理设计高 H 2 O 2选择性 M-N-C SACs 提供了新的见解。
更新日期:2022-06-15
中文翻译:

使用理论动力学计算确定 M–N–C 单原子催化剂上电化学生产 H2O2 的活性趋势
金属-氮-碳 (M-N-C) 单原子催化剂 (SAC) 已显示出在酸性电解质中通过双电子氧还原反应 (2e - ORR) 途径产生 H 2 O 2的高潜力。然而,仍然缺乏有效的动力学计算来揭示 M-N-C SACs 对 2e - ORR 的内在催化性能。在这里,我们结合实验和计算工作来研究M-N-C SAC(M = Co、Fe 和 Mn)的 2e - ORR 活性趋势。实验结果表明,Co-N-C 和 Mn-N-C SAC 有利于 2e - ORR 途径,但 Fe-N-C SAC 有利于 4e -ORR 通路。使用系统动力学计算,我们证明 HOOH* 或 H 2 O* 的解吸是 M-N-C SAC 上 ORR 途径的真正决定性步骤。H 2 O* 和 HOOH* 反应氧中间体 ( E H 2 O* – E HOOH* ) 在金属活性中心上的解吸能差异是说明和预测 M– 的 H 2 O 2选择性的可靠描述符N–C SAC。预测的高 H 2 O 2描述符中 Ni-N-C SAC 的选择性与实验结果一致。本研究阐明了 ORR 对 M-N-C SACs 的作用机制,并确定了 ORR 途径的决定因素,为合理设计高 H 2 O 2选择性 M-N-C SACs 提供了新的见解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号