当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
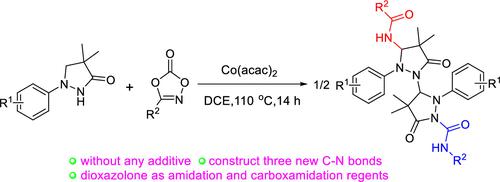
Cobalt(II)-Catalyzed C–H and N–H Functionalization of 1-Arylpyrazolidinones with Dioxazolones as Bifunctional Synthons
Organic Letters ( IF 5.2 ) Pub Date : 2022-06-15 , DOI: 10.1021/acs.orglett.2c01780 Xiliang Han 1 , Chao Pi 1 , Di Hu 1 , Wei Hu 1 , Yangjie Wu 1 , Xiuling Cui 1
Organic Letters ( IF 5.2 ) Pub Date : 2022-06-15 , DOI: 10.1021/acs.orglett.2c01780 Xiliang Han 1 , Chao Pi 1 , Di Hu 1 , Wei Hu 1 , Yangjie Wu 1 , Xiuling Cui 1
Affiliation
|
Dioxazolone has been attractive as an important synthon for a direct C–H amidation through a nitrene intermediate or Curtius rearrangement to form the isocyanate. However, the combination of two reaction models of dioxazolone has not been reported. Herein, a cobalt-catalyzed C–H and N–H functionalization of 1-arylpyrazolidinones with dioxazolones was developed. The dioxazolones acted as an amidated and carboxamidated reagent. Three C–N bonds were formed in a “one-pot” manner, which promoted the requirement of synthetic diversity.
中文翻译:

钴 (II)-催化 1-芳基吡唑烷酮的 C-H 和 N-H 官能化,二恶唑酮作为双功能合成子
二恶唑酮作为一种重要的合成子很有吸引力,它可以通过氮烯中间体直接 C-H 酰胺化或 Curtius 重排形成异氰酸酯。然而,二恶唑酮的两种反应模型的组合尚未见报道。在此,开发了一种钴催化的 C-H 和 N-H 官能化 1-芳基吡唑烷酮与二恶唑酮。二恶唑酮用作酰胺化和羧酰胺化试剂。三个C-N键以“一锅”的方式形成,促进了合成多样性的要求。
更新日期:2022-06-15
中文翻译:

钴 (II)-催化 1-芳基吡唑烷酮的 C-H 和 N-H 官能化,二恶唑酮作为双功能合成子
二恶唑酮作为一种重要的合成子很有吸引力,它可以通过氮烯中间体直接 C-H 酰胺化或 Curtius 重排形成异氰酸酯。然而,二恶唑酮的两种反应模型的组合尚未见报道。在此,开发了一种钴催化的 C-H 和 N-H 官能化 1-芳基吡唑烷酮与二恶唑酮。二恶唑酮用作酰胺化和羧酰胺化试剂。三个C-N键以“一锅”的方式形成,促进了合成多样性的要求。


























 京公网安备 11010802027423号
京公网安备 11010802027423号