当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exploring Pathways and Mechanisms for Dichloroacetonitrile Formation from Typical Amino Compounds during UV/Chlorine Treatment
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2022-06-15 , DOI: 10.1021/acs.est.2c01495 Zhechao Hua 1 , Junfang Li 1 , Zhihong Zhou 2 , Shanshan Zheng 3 , Yifei Zhang 1 , Jingyun Fang 1
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2022-06-15 , DOI: 10.1021/acs.est.2c01495 Zhechao Hua 1 , Junfang Li 1 , Zhihong Zhou 2 , Shanshan Zheng 3 , Yifei Zhang 1 , Jingyun Fang 1
Affiliation
|
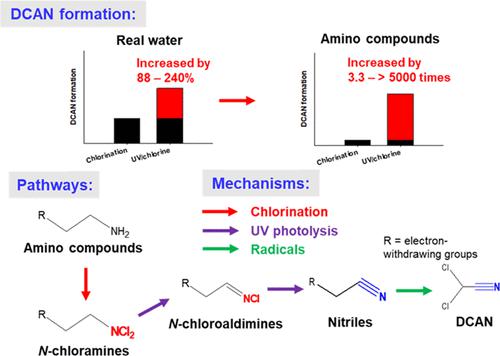
The formation of disinfection byproducts (DBPs) during UV/chlorine treatment, especially nitrogenous DBPs, is not well understood. This study investigated the formation mechanisms for dichloroacetonitrile (DCAN) from typical amino compounds during UV/chlorine treatment. Compared to chlorination, the yields of DCAN increase by 88–240% during UV/chlorine treatment from real waters, while the yields of DCAN from amino compounds increase by 3.3–5724 times. Amino compounds with electron-withdrawing side chains show much higher DCAN formation than those with electron-donating side chains. Phenylethylamine, l- phenylalanine, and l-phenylalanyl-l-phenylalanine were selected to represent amines, amino acids, and peptides, respectively, to investigate the formation pathways for DCAN during UV/chlorine treatment. First, chlorination of amines, amino acids, and peptides rapidly forms N-chloramines via chlorine substitution. Then, UV photolysis but not radicals promotes the transformation from N-chloramines to N-chloroaldimines and then to phenylacetonitrile, with yields of 5.4, 51.0, and 19.8% from chlorinated phenylethylamine, l-phenylalanine, and l-phenylalanyl-l-phenylalanine to phenylacetonitrile, respectively. Finally, phenylacetonitrile is transformed to DCAN with conversion ratios of 14.2–25.6%, which is attributed to radical oxidation, as indicated by scavenging experiments and density functional theory calculations. This study elucidates the pathways and mechanisms for DCAN formation from typical amino compounds during UV/chlorine treatment.
中文翻译:

探索紫外/氯处理过程中典型氨基化合物形成二氯乙腈的途径和机制
紫外线/氯处理过程中消毒副产物 (DBP) 的形成,尤其是含氮 DBP,尚不清楚。本研究调查了典型氨基化合物在紫外/氯处理过程中二氯乙腈 (DCAN) 的形成机制。与氯化相比,在真实水体的紫外/氯处理过程中,DCAN 的产量增加了 88-240%,而氨基化合物的 DCAN 产量增加了 3.3-5724 倍。具有吸电子侧链的氨基化合物显示出比具有给电子侧链的氨基化合物高得多的 DCAN 形成。苯乙胺、l-苯丙氨酸和l-苯丙氨酰-l选择-苯丙氨酸分别代表胺、氨基酸和肽,以研究在紫外线/氯处理过程中 DCAN 的形成途径。首先,胺、氨基酸和肽的氯化通过氯取代迅速形成N-氯胺。然后,UV 光解而非自由基促进从N-氯胺到N-氯醛亚胺再到苯乙腈的转化,氯化苯乙胺、l-苯丙氨酸和l-苯丙氨酰-l的产率分别为 5.4%、51.0% 和 19.8%-苯丙氨酸分别为苯乙腈。最后,如清除实验和密度泛函理论计算所示,苯乙腈以 14.2-25.6% 的转化率转化为 DCAN,这归因于自由基氧化。本研究阐明了在紫外线/氯处理过程中典型氨基化合物形成 DCAN 的途径和机制。
更新日期:2022-06-15
中文翻译:

探索紫外/氯处理过程中典型氨基化合物形成二氯乙腈的途径和机制
紫外线/氯处理过程中消毒副产物 (DBP) 的形成,尤其是含氮 DBP,尚不清楚。本研究调查了典型氨基化合物在紫外/氯处理过程中二氯乙腈 (DCAN) 的形成机制。与氯化相比,在真实水体的紫外/氯处理过程中,DCAN 的产量增加了 88-240%,而氨基化合物的 DCAN 产量增加了 3.3-5724 倍。具有吸电子侧链的氨基化合物显示出比具有给电子侧链的氨基化合物高得多的 DCAN 形成。苯乙胺、l-苯丙氨酸和l-苯丙氨酰-l选择-苯丙氨酸分别代表胺、氨基酸和肽,以研究在紫外线/氯处理过程中 DCAN 的形成途径。首先,胺、氨基酸和肽的氯化通过氯取代迅速形成N-氯胺。然后,UV 光解而非自由基促进从N-氯胺到N-氯醛亚胺再到苯乙腈的转化,氯化苯乙胺、l-苯丙氨酸和l-苯丙氨酰-l的产率分别为 5.4%、51.0% 和 19.8%-苯丙氨酸分别为苯乙腈。最后,如清除实验和密度泛函理论计算所示,苯乙腈以 14.2-25.6% 的转化率转化为 DCAN,这归因于自由基氧化。本研究阐明了在紫外线/氯处理过程中典型氨基化合物形成 DCAN 的途径和机制。


























 京公网安备 11010802027423号
京公网安备 11010802027423号