当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Application of a transient directing strategy in cyclization reactions via C–H activation
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-06-14 , DOI: 10.1039/d2qo00765g Ming Zhang 1, 2 , Zukang Zhong 1, 2 , Lihua Liao 1, 2 , Ai Qin Zhang 3
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-06-14 , DOI: 10.1039/d2qo00765g Ming Zhang 1, 2 , Zukang Zhong 1, 2 , Lihua Liao 1, 2 , Ai Qin Zhang 3
Affiliation
|
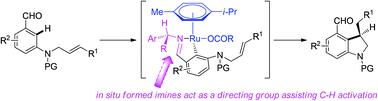
This review introduces the application of a transient directing strategy in cyclization reactions via C–H activation. The types of cyclization reactions include C–H activation/intramolecular C(sp3)–H arylation, C–H activation/intramolecular hydroarylation of alkenes, C–H activation/intramolecular alkene hydroacylation, cyclization through C–C activation and C–H activation in turn, cascade reactions via C–H activation/functionalization and intramolecular nucleophilic addition, cascade reactions via C–H activation/functionalization and intramolecular electrophilic aromatic substitution, and cascade reactions via C–H activation/functionalization and C–N oxidative coupling. These cyclization reactions are catalyzed by Pd, Rh, Ru or Re catalysts. Reactions of the substrates (aldehydes, ketones, a phenol derivative, anilines, etc.) with transient directing groups/reagents (aliphatic amines, aromatic amines, a phosphinite, a secondary amide, an alkyl nitrite, etc.) in situ form directing groups as mono-, bi-, or tridentate ligands assisting C–H activation. Many kinds of carbo-/heterocyclic scaffolds of four-, five-, six-, or seven-membered rings have been constructed. Regio-, chemo- or diastereoselectivities are excellent in most cases. Enantioselectivities are also excellent in some cases where chiral transient directing groups are used. The synthesized cyclic compounds will find applications in pharmaceutical chemistry, material chemistry and synthetic chemistry.
中文翻译:

瞬态导向策略在 C-H 活化环化反应中的应用
本综述介绍了瞬态导向策略通过C-H 活化在环化反应中的应用。环化反应的类型包括C-H活化/分子内C(sp 3 )-H芳基化、C-H活化/烯烃分子内加氢芳基化、C-H活化/分子内烯烃加氢酰化、C-C活化环化和C-H依次活化,通过C-H 活化/功能化和分子内亲核加成的级联反应,通过C-H 活化/功能化和分子内亲电芳族取代的级联反应,以及通过C-H 活化/功能化和 C-N 氧化偶联。这些环化反应由 Pd、Rh、Ru 或 Re 催化剂催化。底物(醛、酮、苯酚衍生物、苯胺等)与瞬时导向基团/试剂(脂肪胺、芳香胺、次膦酸盐、仲酰胺、亚硝酸烷基酯等)的原位反应形成导向基团作为单齿、双齿或三齿配体,协助 C-H 活化。已经构建了多种四元、五元、六元或七元环的碳/杂环支架。在大多数情况下,区域选择性、化学选择性或非对映选择性都非常好。在使用手性瞬态导向基团的某些情况下,对映选择性也很出色。合成的环状化合物将在药物化学、材料化学和合成化学中得到应用。
更新日期:2022-06-14
中文翻译:

瞬态导向策略在 C-H 活化环化反应中的应用
本综述介绍了瞬态导向策略通过C-H 活化在环化反应中的应用。环化反应的类型包括C-H活化/分子内C(sp 3 )-H芳基化、C-H活化/烯烃分子内加氢芳基化、C-H活化/分子内烯烃加氢酰化、C-C活化环化和C-H依次活化,通过C-H 活化/功能化和分子内亲核加成的级联反应,通过C-H 活化/功能化和分子内亲电芳族取代的级联反应,以及通过C-H 活化/功能化和 C-N 氧化偶联。这些环化反应由 Pd、Rh、Ru 或 Re 催化剂催化。底物(醛、酮、苯酚衍生物、苯胺等)与瞬时导向基团/试剂(脂肪胺、芳香胺、次膦酸盐、仲酰胺、亚硝酸烷基酯等)的原位反应形成导向基团作为单齿、双齿或三齿配体,协助 C-H 活化。已经构建了多种四元、五元、六元或七元环的碳/杂环支架。在大多数情况下,区域选择性、化学选择性或非对映选择性都非常好。在使用手性瞬态导向基团的某些情况下,对映选择性也很出色。合成的环状化合物将在药物化学、材料化学和合成化学中得到应用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号