当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unified Total Synthesis of Tetracyclic Diquinane Lycopodium Alkaloids (+)-Paniculatine, (−)-Magellanine, and (+)-Magellaninone
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2022-06-14 , DOI: 10.1021/acs.joc.2c00871 Bing-Bing Huang 1 , Kaiyu Lei 1 , Lin-Rui Zhong 1 , Xiaoliang Yang 1 , Zhu-Jun Yao 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2022-06-14 , DOI: 10.1021/acs.joc.2c00871 Bing-Bing Huang 1 , Kaiyu Lei 1 , Lin-Rui Zhong 1 , Xiaoliang Yang 1 , Zhu-Jun Yao 1
Affiliation
|
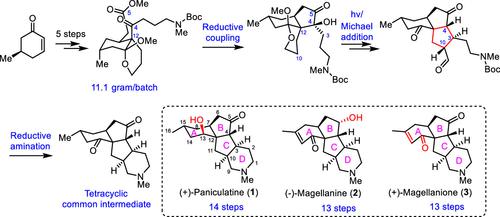
A unified route for the total synthesis of three tetracyclic diquinane Lycopodium alkaloids (+)-paniculatine, (−)-magellanine, and (+)-magellaninone has been accomplished in 13–14 overall steps based on late-stage diverse transformations from an advanced tetracyclic common intermediate. In the established synthesis, quick formation of the two five-membered rings was efficiently achieved by an intramolecular reductive coupling of ketone–carbonyl and ester–carbonyl and an organocatalytic intramolecular Michael addition of aldehyde-derived enamine to an internal enone functionality with satisfactory redox and step economies and excellent stereoselectivities, providing the requisite tricyclic carbo-framework possessing multiple dense stereogenic centers, and an intramolecular reductive amination finally furnished the essential piperidine ring.
中文翻译:

四环二喹烷石松碱 (+)-Paniculatine、(-)-Magellanine 和 (+)-Magellaninone 的统一全合成
三种四环二喹烷石松全合成的统一路线生物碱 (+)-paniculatine、(-)-magellanine 和 (+)-magellaninone 基于来自高级四环常见中间体的后期多样化转化,在 13-14 个整体步骤中完成。在已建立的合成中,两个五元环的快速形成是通过酮-羰基和酯-羰基的分子内还原偶联以及醛衍生烯胺的有机催化分子内迈克尔加成到具有令人满意的氧化还原和阶梯经济和出色的立体选择性,提供了具有多个致密立体中心的必要三环碳骨架,并且分子内还原胺化最终提供了必要的哌啶环。
更新日期:2022-06-14
中文翻译:

四环二喹烷石松碱 (+)-Paniculatine、(-)-Magellanine 和 (+)-Magellaninone 的统一全合成
三种四环二喹烷石松全合成的统一路线生物碱 (+)-paniculatine、(-)-magellanine 和 (+)-magellaninone 基于来自高级四环常见中间体的后期多样化转化,在 13-14 个整体步骤中完成。在已建立的合成中,两个五元环的快速形成是通过酮-羰基和酯-羰基的分子内还原偶联以及醛衍生烯胺的有机催化分子内迈克尔加成到具有令人满意的氧化还原和阶梯经济和出色的立体选择性,提供了具有多个致密立体中心的必要三环碳骨架,并且分子内还原胺化最终提供了必要的哌啶环。


























 京公网安备 11010802027423号
京公网安备 11010802027423号