当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and hydrogenation of polycyclic aromatic hydrocarbon-substituted diborenes via uncatalysed hydrogenative B–C bond cleavage
Chemical Science ( IF 8.4 ) Pub Date : 2022-06-14 , DOI: 10.1039/d2sc02515a Alexander Okorn 1, 2 , Arumugam Jayaraman 1, 2 , Lukas Englert 1, 2 , Merle Arrowsmith 1, 2 , Theresa Swoboda 1, 2 , Jeanette Weigelt 1, 2 , Carina Brunecker 1, 2 , Merlin Hess 1, 2 , Anna Lamprecht 1, 2 , Carsten Lenczyk 1, 2 , Maximilian Rang 1, 2 , Holger Braunschweig 1, 2
Chemical Science ( IF 8.4 ) Pub Date : 2022-06-14 , DOI: 10.1039/d2sc02515a Alexander Okorn 1, 2 , Arumugam Jayaraman 1, 2 , Lukas Englert 1, 2 , Merle Arrowsmith 1, 2 , Theresa Swoboda 1, 2 , Jeanette Weigelt 1, 2 , Carina Brunecker 1, 2 , Merlin Hess 1, 2 , Anna Lamprecht 1, 2 , Carsten Lenczyk 1, 2 , Maximilian Rang 1, 2 , Holger Braunschweig 1, 2
Affiliation
|
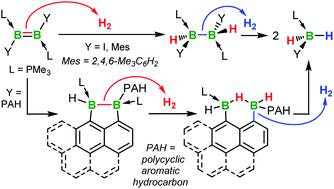
The classical route to the PMe3-stabilised polycyclic aromatic hydrocarbon (PAH)-substituted diborenes B2Ar2(PMe3)2 (Ar = 9-phenanthryl 7-Phen; Ar = 1-pyrenyl 7-Pyr) via the corresponding 1,2-diaryl-1,2-dimethoxydiborane(4) precursors, B2Ar2(OMe)2, is marred by the systematic decomposition of the latter to BAr(OMe)2 during reaction workup. Calculations suggest this results from the absence of a second ortho-substituent on the boron-bound aryl rings, which enables their free rotation and exposes the B–B bond to nucleophilic attack. 7-Phen and 7-Pyr are obtained by the reduction of the corresponding 1,2-diaryl-1,2-dichlorodiborane precursors, B2Ar2Cl2(PMe3)2, obtained from the SMe2 adducts, which are synthesised by direct NMe2–Cl exchange at B2Ar2(NMe2)2 with (Me2S)BCl3. The low-lying π* molecular orbitals (MOs) located on the PAH substituents of 7-Phen and 7-Pyr intercalate between the B–B-based π and π* MOs, leading to a relatively small HOMO–LUMO gap of 3.20 and 2.72 eV, respectively. Under vacuum or at high temperature 7-Phen and 7-Pyr undergo intramolecular hydroarylation of the B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B bond to yield 1,2-dihydronaphtho[1,8-cd][1,2]diborole derivatives. Hydrogenation of 7-Phen, 7-Pyr and their 9-anthryl and mesityl analogues III and II, respectively, results in all cases in splitting of the B–B bond and isolation of the monoboranes (Me3P)BArH2. NMR-spectroscopic monitoring of the reactions, solid-state structures of isolated reaction intermediates and computational mechanistic analyses show that the hydrogenation of the three PAH-substituted diborenes proceeds via a different pathway to that of the dimesityldiborene. Rather than occurring exclusively at the B–B bond, hydrogenation of 7-Ar and III proceeds via a hydroarylated intermediate, which undergoes one B–B bond-centered H2 addition, followed by hydrogenation of the endocyclic B–C bond resulting from hydroarylation, making the latter effectively reversible.
B bond to yield 1,2-dihydronaphtho[1,8-cd][1,2]diborole derivatives. Hydrogenation of 7-Phen, 7-Pyr and their 9-anthryl and mesityl analogues III and II, respectively, results in all cases in splitting of the B–B bond and isolation of the monoboranes (Me3P)BArH2. NMR-spectroscopic monitoring of the reactions, solid-state structures of isolated reaction intermediates and computational mechanistic analyses show that the hydrogenation of the three PAH-substituted diborenes proceeds via a different pathway to that of the dimesityldiborene. Rather than occurring exclusively at the B–B bond, hydrogenation of 7-Ar and III proceeds via a hydroarylated intermediate, which undergoes one B–B bond-centered H2 addition, followed by hydrogenation of the endocyclic B–C bond resulting from hydroarylation, making the latter effectively reversible.
中文翻译:

通过未催化的氢化B-C键断裂合成和氢化多环芳烃取代的二硼烯
通过相应的 1 获得 PMe 3稳定的多环芳烃 (PAH) 取代的二硼烯 B 2 Ar 2 (PMe 3 ) 2 (Ar = 9-菲基7-Phen ; Ar = 1-芘基 7-Pyr )的经典路线,2-diaryl-1,2-dimethoxydiborane(4) 前体 B 2 Ar 2 (OMe ) 2在反应后处理过程中被系统分解为 BAr(OMe) 2 所破坏。计算表明这是由于没有第二个邻位-硼结合的芳环上的取代基,使其能够自由旋转并使 B-B 键暴露于亲核攻击。7-Phen和7-Pyr是通过还原相应的 1,2-diaryl-1,2-dichlorodiborane 前体 B 2 Ar 2 Cl 2 (PMe 3 ) 2得到的,它们是由合成的 SMe 2加合物获得的通过在B 2 Ar 2 (NMe 2 ) 2与(Me 2 S)BCl 3直接交换NMe 2 -Cl 。位于 PAH 取代基上的低位 π* 分子轨道 (MOs)7-Phen和7-Pyr插入基于 B-B 的 π 和 π* MOs 之间,导致相对较小的 HOMO-LUMO 间隙分别为 3.20 和 2.72 eV。在真空或高温下, 7-Phen和7-Pyr进行 B![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B 键的分子内氢化芳基化,生成 1,2-二氢萘并[1,8- cd ][1,2]二硼烷衍生物。7-Phen、7-Pyr和它们的 9-蒽基和异三叉戟类似物III和II的加氢反应在所有情况下均导致 B-B 键断裂和单硼烷 (Me 3 P)BArH 2的分离. 反应的 NMR 光谱监测、分离的反应中间体的固态结构和计算机理分析表明,三种 PAH 取代的二硼烯的氢化通过与二苯甲基二硼烯不同的途径进行。7-Ar和III的氢化不仅仅发生在 B-B 键上,而是通过氢化中间体进行,该中间体经历一个以 B-B 键为中心的 H 2加成,然后氢化由氢化芳基化产生的环内 B-C 键,使后者有效地可逆。
B 键的分子内氢化芳基化,生成 1,2-二氢萘并[1,8- cd ][1,2]二硼烷衍生物。7-Phen、7-Pyr和它们的 9-蒽基和异三叉戟类似物III和II的加氢反应在所有情况下均导致 B-B 键断裂和单硼烷 (Me 3 P)BArH 2的分离. 反应的 NMR 光谱监测、分离的反应中间体的固态结构和计算机理分析表明,三种 PAH 取代的二硼烯的氢化通过与二苯甲基二硼烯不同的途径进行。7-Ar和III的氢化不仅仅发生在 B-B 键上,而是通过氢化中间体进行,该中间体经历一个以 B-B 键为中心的 H 2加成,然后氢化由氢化芳基化产生的环内 B-C 键,使后者有效地可逆。
更新日期:2022-06-14
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B bond to yield 1,2-dihydronaphtho[1,8-cd][1,2]diborole derivatives. Hydrogenation of 7-Phen, 7-Pyr and their 9-anthryl and mesityl analogues III and II, respectively, results in all cases in splitting of the B–B bond and isolation of the monoboranes (Me3P)BArH2. NMR-spectroscopic monitoring of the reactions, solid-state structures of isolated reaction intermediates and computational mechanistic analyses show that the hydrogenation of the three PAH-substituted diborenes proceeds via a different pathway to that of the dimesityldiborene. Rather than occurring exclusively at the B–B bond, hydrogenation of 7-Ar and III proceeds via a hydroarylated intermediate, which undergoes one B–B bond-centered H2 addition, followed by hydrogenation of the endocyclic B–C bond resulting from hydroarylation, making the latter effectively reversible.
B bond to yield 1,2-dihydronaphtho[1,8-cd][1,2]diborole derivatives. Hydrogenation of 7-Phen, 7-Pyr and their 9-anthryl and mesityl analogues III and II, respectively, results in all cases in splitting of the B–B bond and isolation of the monoboranes (Me3P)BArH2. NMR-spectroscopic monitoring of the reactions, solid-state structures of isolated reaction intermediates and computational mechanistic analyses show that the hydrogenation of the three PAH-substituted diborenes proceeds via a different pathway to that of the dimesityldiborene. Rather than occurring exclusively at the B–B bond, hydrogenation of 7-Ar and III proceeds via a hydroarylated intermediate, which undergoes one B–B bond-centered H2 addition, followed by hydrogenation of the endocyclic B–C bond resulting from hydroarylation, making the latter effectively reversible.
中文翻译:

通过未催化的氢化B-C键断裂合成和氢化多环芳烃取代的二硼烯
通过相应的 1 获得 PMe 3稳定的多环芳烃 (PAH) 取代的二硼烯 B 2 Ar 2 (PMe 3 ) 2 (Ar = 9-菲基7-Phen ; Ar = 1-芘基 7-Pyr )的经典路线,2-diaryl-1,2-dimethoxydiborane(4) 前体 B 2 Ar 2 (OMe ) 2在反应后处理过程中被系统分解为 BAr(OMe) 2 所破坏。计算表明这是由于没有第二个邻位-硼结合的芳环上的取代基,使其能够自由旋转并使 B-B 键暴露于亲核攻击。7-Phen和7-Pyr是通过还原相应的 1,2-diaryl-1,2-dichlorodiborane 前体 B 2 Ar 2 Cl 2 (PMe 3 ) 2得到的,它们是由合成的 SMe 2加合物获得的通过在B 2 Ar 2 (NMe 2 ) 2与(Me 2 S)BCl 3直接交换NMe 2 -Cl 。位于 PAH 取代基上的低位 π* 分子轨道 (MOs)7-Phen和7-Pyr插入基于 B-B 的 π 和 π* MOs 之间,导致相对较小的 HOMO-LUMO 间隙分别为 3.20 和 2.72 eV。在真空或高温下, 7-Phen和7-Pyr进行 B
![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B 键的分子内氢化芳基化,生成 1,2-二氢萘并[1,8- cd ][1,2]二硼烷衍生物。7-Phen、7-Pyr和它们的 9-蒽基和异三叉戟类似物III和II的加氢反应在所有情况下均导致 B-B 键断裂和单硼烷 (Me 3 P)BArH 2的分离. 反应的 NMR 光谱监测、分离的反应中间体的固态结构和计算机理分析表明,三种 PAH 取代的二硼烯的氢化通过与二苯甲基二硼烯不同的途径进行。7-Ar和III的氢化不仅仅发生在 B-B 键上,而是通过氢化中间体进行,该中间体经历一个以 B-B 键为中心的 H 2加成,然后氢化由氢化芳基化产生的环内 B-C 键,使后者有效地可逆。
B 键的分子内氢化芳基化,生成 1,2-二氢萘并[1,8- cd ][1,2]二硼烷衍生物。7-Phen、7-Pyr和它们的 9-蒽基和异三叉戟类似物III和II的加氢反应在所有情况下均导致 B-B 键断裂和单硼烷 (Me 3 P)BArH 2的分离. 反应的 NMR 光谱监测、分离的反应中间体的固态结构和计算机理分析表明,三种 PAH 取代的二硼烯的氢化通过与二苯甲基二硼烯不同的途径进行。7-Ar和III的氢化不仅仅发生在 B-B 键上,而是通过氢化中间体进行,该中间体经历一个以 B-B 键为中心的 H 2加成,然后氢化由氢化芳基化产生的环内 B-C 键,使后者有效地可逆。


























 京公网安备 11010802027423号
京公网安备 11010802027423号