Hydrometallurgy ( IF 4.7 ) Pub Date : 2022-06-12 , DOI: 10.1016/j.hydromet.2022.105914 Ruibing Bai , Junfeng Wang , Daoguang Wang , Junjie Cui , Yanqiang Zhang
|
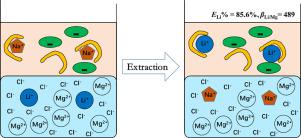
In view of the complex problems for the ionic liquids (ILs) recovery with the system containing ILs and TBP, this work proposed a NaNTf2-based synergistic extraction system, NaNTf2 + TBP, which could realize the effective reutilization of the extraction system without reducing the lithium separation ability. Under the optimal extraction parameters, the extraction efficiency of Li+ exceeded 98%, while that of Mg2+ was only ~1%. The results of mass spectrometry (ESI-MS) and slope analysis showed that two TBP molecules involved in the extraction process of Li+, and the Li+ extraction mechanism obeyed the cation-exchange process. According to the red shift of the FT − IR peak of P O in TBP and the chemical shift changes of P in 31P NMR, the interaction strength between TBP and different metal ion followed the sequence: ETBP−H >> ETBP−Li > ETBP−Mg > ETBP−K > ETBP−Na. It was also found that after a three−stage countercurrent extraction process, [Li+]org was 0.42 g L−1, and the total recovery efficiency of Li+ was about 98%. The saturated extraction capacity for Li+ could be as high as 5.4 g L−1, and Li+ could be stripped with the aqueous solution containing 0.2 mol L-1 HCl. In addition, the extraction efficiency of Li+ was almost not attenuated after 6 extraction cycles. This new extraction system shows a certain industrial application prospect for Li+ separation from the salt lake bine with high Mg/Li ratio.
O in TBP and the chemical shift changes of P in 31P NMR, the interaction strength between TBP and different metal ion followed the sequence: ETBP−H >> ETBP−Li > ETBP−Mg > ETBP−K > ETBP−Na. It was also found that after a three−stage countercurrent extraction process, [Li+]org was 0.42 g L−1, and the total recovery efficiency of Li+ was about 98%. The saturated extraction capacity for Li+ could be as high as 5.4 g L−1, and Li+ could be stripped with the aqueous solution containing 0.2 mol L-1 HCl. In addition, the extraction efficiency of Li+ was almost not attenuated after 6 extraction cycles. This new extraction system shows a certain industrial application prospect for Li+ separation from the salt lake bine with high Mg/Li ratio.
中文翻译:

使用 NaNTf2 和 TBP 离子交换从高 Mg/Li 比盐湖卤水中回收锂
针对含ILs和TBP的体系在离子液体(ILs)回收中存在的复杂问题,本工作提出了一种基于NaNTf 2的协同萃取体系NaNTf 2 + TBP,该体系无需降低锂分离能力。在最优提取参数下,Li +的提取效率超过98%,而Mg 2+的提取效率仅为~1%。质谱(ESI-MS)和斜率分析结果表明,有两个TBP分子参与了Li + 的提取过程,Li +的提取机理服从阳离子交换过程。根据 P 的 FT - IR 峰的红移 TBP中的O和31 P NMR中P的化学位移变化,TBP与不同金属离子的相互作用强度依次为:E TBP-H >> E TBP-Li > E TBP-Mg > E TBP-K > E TBP-Na。还发现经过三级逆流萃取过程,[Li + ] org为0.42 g L -1,Li +的总回收率约为98%。Li +的饱和萃取容量可高达 5.4 g L -1,而 Li +可用含 0.2 mol L -1 HCl的水溶液汽提。此外,Li +的萃取效率在 6 次萃取循环后几乎没有衰减。这种新的萃取系统在高镁锂比盐湖沼液中分离锂离子具有一定的工业应用前景。
TBP中的O和31 P NMR中P的化学位移变化,TBP与不同金属离子的相互作用强度依次为:E TBP-H >> E TBP-Li > E TBP-Mg > E TBP-K > E TBP-Na。还发现经过三级逆流萃取过程,[Li + ] org为0.42 g L -1,Li +的总回收率约为98%。Li +的饱和萃取容量可高达 5.4 g L -1,而 Li +可用含 0.2 mol L -1 HCl的水溶液汽提。此外,Li +的萃取效率在 6 次萃取循环后几乎没有衰减。这种新的萃取系统在高镁锂比盐湖沼液中分离锂离子具有一定的工业应用前景。


























 京公网安备 11010802027423号
京公网安备 11010802027423号