当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tunable Redox Cycle and Enhanced π-Complexation in Acetylene Hydrochlorination over RuCu Catalysts
ACS Catalysis ( IF 12.9 ) Pub Date : 2022-06-13 , DOI: 10.1021/acscatal.2c01559 Yurui Fan 1 , Haomiao Xu 1 , Zhisong Liu 1, 2 , Songyuan Sun 1 , Wenjun Huang 1 , Zan Qu 1 , Naiqiang Yan 1, 3
ACS Catalysis ( IF 12.9 ) Pub Date : 2022-06-13 , DOI: 10.1021/acscatal.2c01559 Yurui Fan 1 , Haomiao Xu 1 , Zhisong Liu 1, 2 , Songyuan Sun 1 , Wenjun Huang 1 , Zan Qu 1 , Naiqiang Yan 1, 3
Affiliation
|
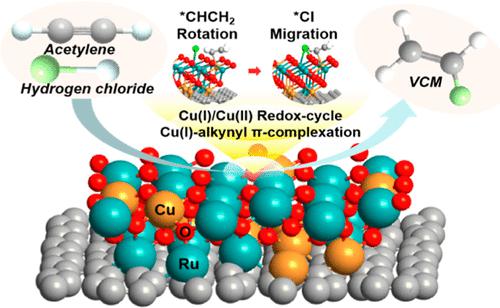
Acetylene hydrochlorination is the core reaction in vinyl chloride monomer (VCM) production. Ruthenium chloride (RuClx) has emerged as a promising nonmercury alternative to replace the supported mercuric chloride (HgCl2) catalysts. However, it has some obstacles such as low activity, coke deposition, and over-reduction of active ruthenium (Ru) species. In this study, we found that the cooperation of Cu(X) (X = 0, I, and II) enhanced both the acetylene (C2H2) conversion efficiency (>96%) and VCM selectivity (>97%). Notably, the anchored Cu(I) ions can promote the rapid C2H2 molecule adsorption through Cu(I)-alkynyl π-complexation with the side-on mode. Furthermore, the one-electron complementary redox cycle of Cu(I)/Cu(II) pairs contributed to the Ru(III)/Ru(IV) cycle in the catalytic process. Density functional theory calculation results indicated that the Ru–O–Cu coordination sites played a crucial role in the catalytic activity of the hydrochlorination reaction, and the migration of Cl* was identified as the rate-limiting step of the entire catalytic pathway. The bimetallic RuCu/AC catalyst guaranteed the continuity and high efficiency of the reaction. Moreover, for the long-term catalytic reaction over 100 h, the C2H2 conversion efficiency was only decreased by 1.46% due to the restriction of in situ coke formation. These findings provide guidance for designing efficient Ru-based catalysts and solutions for engineering applications to replace existing mercury-contained catalysts.
中文翻译:

RuCu 催化剂上乙炔氢氯化反应的可调谐氧化还原循环和增强的 π 络合
乙炔氢氯化是氯乙烯单体(VCM)生产中的核心反应。氯化钌 (RuCl x ) 已成为替代负载型氯化汞 (HgCl 2 ) 催化剂的有前途的非汞替代品。然而,它存在一些障碍,例如活性低、积炭和活性钌(Ru)物种的过度还原。在这项研究中,我们发现 Cu(X)(X = 0、I 和 II)的合作提高了乙炔(C 2 H 2)的转化效率(>96%)和 VCM 的选择性(>97%)。值得注意的是,锚定的 Cu(I) 离子可以促进快速 C 2 H 2通过 Cu(I)-炔基 π-络合与侧向模式的分子吸附。此外,Cu(I)/Cu(II) 对的单电子互补氧化还原循环有助于催化过程中的 Ru(III)/Ru(IV) 循环。密度泛函理论计算结果表明,Ru-O-Cu配位点对氢氯化反应的催化活性起着至关重要的作用,Cl*的迁移被确定为整个催化途径的限速步骤。双金属RuCu/AC催化剂保证了反应的连续性和高效性。此外,对于超过 100 小时的长期催化反应,C 2 H 2由于原位焦炭形成的限制,转化效率仅降低了 1.46%。这些发现为设计高效的钌基催化剂和工程应用解决方案以替代现有的含汞催化剂提供了指导。
更新日期:2022-06-13
中文翻译:

RuCu 催化剂上乙炔氢氯化反应的可调谐氧化还原循环和增强的 π 络合
乙炔氢氯化是氯乙烯单体(VCM)生产中的核心反应。氯化钌 (RuCl x ) 已成为替代负载型氯化汞 (HgCl 2 ) 催化剂的有前途的非汞替代品。然而,它存在一些障碍,例如活性低、积炭和活性钌(Ru)物种的过度还原。在这项研究中,我们发现 Cu(X)(X = 0、I 和 II)的合作提高了乙炔(C 2 H 2)的转化效率(>96%)和 VCM 的选择性(>97%)。值得注意的是,锚定的 Cu(I) 离子可以促进快速 C 2 H 2通过 Cu(I)-炔基 π-络合与侧向模式的分子吸附。此外,Cu(I)/Cu(II) 对的单电子互补氧化还原循环有助于催化过程中的 Ru(III)/Ru(IV) 循环。密度泛函理论计算结果表明,Ru-O-Cu配位点对氢氯化反应的催化活性起着至关重要的作用,Cl*的迁移被确定为整个催化途径的限速步骤。双金属RuCu/AC催化剂保证了反应的连续性和高效性。此外,对于超过 100 小时的长期催化反应,C 2 H 2由于原位焦炭形成的限制,转化效率仅降低了 1.46%。这些发现为设计高效的钌基催化剂和工程应用解决方案以替代现有的含汞催化剂提供了指导。



























 京公网安备 11010802027423号
京公网安备 11010802027423号