Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Epitaxial Electrocrystallization of Magnesium via Synergy of Magnesiophilic Interface, Lattice Matching, and Electrostatic Confinement
ACS Nano ( IF 17.1 ) Pub Date : 2022-06-13 , DOI: 10.1021/acsnano.2c04135 Jing Liu 1, 2 , Jinlei Zhang 1 , Zhonghua Zhang 1, 3 , Aobing Du 3 , Shanmu Dong 3 , Zhenfang Zhou 1 , Xiaosong Guo 1 , Qingfu Wang 4 , Zhenjiang Li 1 , Guicun Li 1 , Guanglei Cui 3
ACS Nano ( IF 17.1 ) Pub Date : 2022-06-13 , DOI: 10.1021/acsnano.2c04135 Jing Liu 1, 2 , Jinlei Zhang 1 , Zhonghua Zhang 1, 3 , Aobing Du 3 , Shanmu Dong 3 , Zhenfang Zhou 1 , Xiaosong Guo 1 , Qingfu Wang 4 , Zhenjiang Li 1 , Guicun Li 1 , Guanglei Cui 3
Affiliation
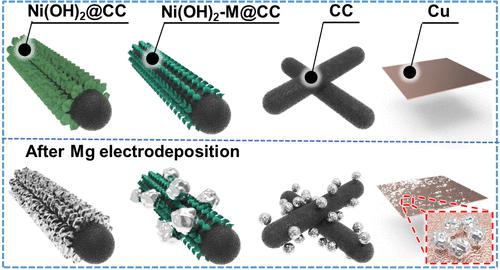
|
Rechargeable magnesium batteries are particularly advantageous for renewable energy storage systems. However, the inhomogeneous Mg electrodeposits greatly shorten their cycle life under practical conditions. Herein, the epitaxial electrocrystallization of Mg on a three-dimensional magnesiophilic host is implemented via the synergy of a magnesiophilic interface, lattice matching, and electrostatic confinement effects. The vertically aligned nickel hydroxide nanosheet arrays grown on carbon cloth (abbreviated as “Ni(OH)2@CC”) have been delicately designed, which satisfy the essential prerequisite of a low lattice geometrical misfit with Mg (about 2.8%) to realize epitaxial electrocrystallization. Simultaneously, the ionic crystal nature of Ni(OH)2 displays a periodic and hillock-like electrostatic potential field over its exposed facets, which can precisely capture and confine the reduced Mg0 species onto the local electron-enriched sites at the atomic level. The Ni(OH)2@CC substrate undergoes sequential Mg-ion intercalation, underpotential deposition, and electrocrystallization processes, during which the uniform, lamellar Mg electrodeposits with a locked crystallographic orientation are formed. Under practical conditions (10 mA cm–2 and 10 mAh cm–2), the Ni(OH)2@CC substrate exhibits stable Mg stripping/plating cycle performances over 600 h, 2 orders of magnitude longer than those of the pristine copper foil and carbon cloth substrates.
中文翻译:

通过亲镁界面、晶格匹配和静电限制的协同作用实现镁的外延电结晶
可充电镁电池特别有利于可再生能源存储系统。然而,不均匀的镁电沉积物在实际条件下大大缩短了它们的循环寿命。在此,镁在三维亲镁主体上的外延电结晶是通过亲镁界面、晶格匹配和静电限制效应的协同作用实现的。在碳布(简称“Ni(OH) 2 @CC”)上生长的垂直排列的氢氧化镍纳米片阵列经过精心设计,满足了与Mg(约2.8%)低晶格几何错配实现外延的必要条件电结晶。同时,Ni(OH) 2的离子晶体性质 displays a periodic and hillock-like electrostatic potential field over its exposed facets, which can precisely capture and confine the reduced Mg0 species onto the local electron-enriched sites at the atomic level. The Ni(OH)2@CC substrate undergoes sequential Mg-ion intercalation, underpotential deposition, and electrocrystallization processes, during which the uniform, lamellar Mg electrodeposits with a locked crystallographic orientation are formed. Under practical conditions (10 mA cm–2 and 10 mAh cm–2), the Ni(OH)2@CC substrate exhibits stable Mg stripping/plating cycle performances over 600 h, 2 orders of magnitude longer than those of the pristine copper foil and carbon cloth substrates.
更新日期:2022-06-13
中文翻译:

通过亲镁界面、晶格匹配和静电限制的协同作用实现镁的外延电结晶
可充电镁电池特别有利于可再生能源存储系统。然而,不均匀的镁电沉积物在实际条件下大大缩短了它们的循环寿命。在此,镁在三维亲镁主体上的外延电结晶是通过亲镁界面、晶格匹配和静电限制效应的协同作用实现的。在碳布(简称“Ni(OH) 2 @CC”)上生长的垂直排列的氢氧化镍纳米片阵列经过精心设计,满足了与Mg(约2.8%)低晶格几何错配实现外延的必要条件电结晶。同时,Ni(OH) 2的离子晶体性质 displays a periodic and hillock-like electrostatic potential field over its exposed facets, which can precisely capture and confine the reduced Mg0 species onto the local electron-enriched sites at the atomic level. The Ni(OH)2@CC substrate undergoes sequential Mg-ion intercalation, underpotential deposition, and electrocrystallization processes, during which the uniform, lamellar Mg electrodeposits with a locked crystallographic orientation are formed. Under practical conditions (10 mA cm–2 and 10 mAh cm–2), the Ni(OH)2@CC substrate exhibits stable Mg stripping/plating cycle performances over 600 h, 2 orders of magnitude longer than those of the pristine copper foil and carbon cloth substrates.



























 京公网安备 11010802027423号
京公网安备 11010802027423号