当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Asymmetric difluorocarbonylation reactions of non-active imines catalyzed by Bi(OAc)3/chiral phosphoric acid
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-06-14 , DOI: 10.1039/d2qo00775d Yu-Liang Pan 1 , Ying-Bo Shao 1 , Zhen Liu 1 , Han-Liang Zheng 1 , Liu Cai 1 , Hai-Chang Zhang 2 , Xin Li 1, 3
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-06-14 , DOI: 10.1039/d2qo00775d Yu-Liang Pan 1 , Ying-Bo Shao 1 , Zhen Liu 1 , Han-Liang Zheng 1 , Liu Cai 1 , Hai-Chang Zhang 2 , Xin Li 1, 3
Affiliation
|
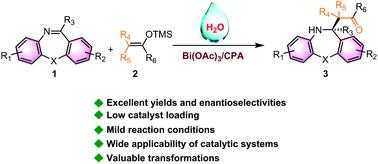
Difluorinated carbonyl has been identified as the basic skeleton of multitudinous biologically active molecules. However, development of strategies for asymmetrically introducing such an important moiety remains a formidable challenge. Herein, we reported a practical and efficient asymmetric difluorocarbonylation reaction of non-active imines with difluoroenoxysilane. In the presence of Bi(OAc)3/chiral phosphoric acid (2 mol%), excellent efficiency (up to 99%) and high enantioselectivity (up to 99 : 1 e.r.) were obtained. The reaction could be scaled up, and the synthetic utility of the desired chiral difluorocarbonylation product was proved by diverse transformations. In addition, the catalytic strategy can also be well applied to other imines, oxocarbenium ions and monofluorinated enol silyl ether. A possible mechanism is proposed to illustrate the reaction process and density functional theory calculation is conducted to interpret the enantioselectivity.
中文翻译:

Bi(OAc)3/手性磷酸催化非活性亚胺的不对称二氟羰基化反应
二氟化羰基已被确定为众多生物活性分子的基本骨架。然而,制定不对称引入如此重要部分的策略仍然是一项艰巨的挑战。在此,我们报道了一种实用且有效的非活性亚胺与二氟烯氧基硅烷的不对称二氟羰基化反应。在 Bi(OAc) 3存在下获得了手性磷酸(2 mol%)、优异的效率(高达 99%)和高对映选择性(高达 99 : 1 er)。该反应可以扩大规模,并且通过多种转化证明了所需手性二氟羰基化产物的合成效用。此外,该催化策略还可以很好地应用于其他亚胺、氧代碳鎓离子和单氟化烯醇甲硅烷基醚。提出了一种可能的机理来说明反应过程,并进行了密度泛函理论计算来解释对映选择性。
更新日期:2022-06-14
中文翻译:

Bi(OAc)3/手性磷酸催化非活性亚胺的不对称二氟羰基化反应
二氟化羰基已被确定为众多生物活性分子的基本骨架。然而,制定不对称引入如此重要部分的策略仍然是一项艰巨的挑战。在此,我们报道了一种实用且有效的非活性亚胺与二氟烯氧基硅烷的不对称二氟羰基化反应。在 Bi(OAc) 3存在下获得了手性磷酸(2 mol%)、优异的效率(高达 99%)和高对映选择性(高达 99 : 1 er)。该反应可以扩大规模,并且通过多种转化证明了所需手性二氟羰基化产物的合成效用。此外,该催化策略还可以很好地应用于其他亚胺、氧代碳鎓离子和单氟化烯醇甲硅烷基醚。提出了一种可能的机理来说明反应过程,并进行了密度泛函理论计算来解释对映选择性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号