当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Improvement of the hydrogen storage performance of t-graphene-like two-dimensional boron nitride upon selected lithium decoration
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2022-06-13 , DOI: 10.1039/d2cp00480a Majid El Kassaoui 1 , Marwan Lakhal 2 , Abdelilah Benyoussef 1, 3 , Abdallah El Kenz 1 , Mohammed Loulidi 1 , Omar Mounkachi 1, 4, 5
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2022-06-13 , DOI: 10.1039/d2cp00480a Majid El Kassaoui 1 , Marwan Lakhal 2 , Abdelilah Benyoussef 1, 3 , Abdallah El Kenz 1 , Mohammed Loulidi 1 , Omar Mounkachi 1, 4, 5
Affiliation
|
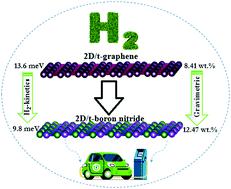
In recent years, search for applicable bidimensional (2D) hydrogen storage materials with high capacity and excellent H2 physisorption properties has attracted considerable attention from scientists and researchers. According to the rational design, and using first-principles calculations, we propose a t-graphene-like boron nitride monolayer (t-B4N4) for hydrogen storage application by replacing C atoms in t-graphene with B and N atoms. The thermal stability and polarization mechanisms of lithium atoms adsorbed at the center of octagons on the t-B4N4 system were evaluated at 300 K using ab initio molecular dynamics (AIMD) calculations. Moreover, Li-decorated double-sided t-B4N4 can store up to 32H2 molecules with an average hydrogen adsorption energy of 0.217 eV per H2 and a maximum hydrogen storage capacity of 12.47 wt%. The reversibility of adsorbed hydrogen was checked and the calculated desorption temperature was 161 K, much higher than the critical point for hydrogen. Based on diffusion barriers, the H2 molecule diffusion kinetics is faster on the t-B4N4 surface than that on t-graphene and graphene.
中文翻译:

选择锂修饰后类 t-石墨烯二维氮化硼的储氢性能改善
近年来,寻找具有高容量和优异H 2物理吸附性能的适用二维(2D)储氢材料引起了科学家和研究人员的广泛关注。根据合理的设计,并使用第一性原理计算,我们提出了一种用于储氢应用的t-石墨烯类氮化硼单层(tB 4 N 4 ),将t-石墨烯中的C原子替换为B和N原子。使用从头算分子动力学 (AIMD) 计算在 300 K 下评估了 tB 4 N 4系统上八边形中心吸附的锂原子的热稳定性和极化机制。此外,锂装饰的双面 tB4 N 4可以储存多达32个H 2分子,平均氢吸附能为0.217 eV/H 2,最大储氢容量为12.47 wt%。检查了吸附氢的可逆性,计算出的解吸温度为 161 K,远高于氢的临界点。基于扩散势垒, tB 4 N 4表面的H 2分子扩散动力学比t-石墨烯和石墨烯上的快。
更新日期:2022-06-13
中文翻译:

选择锂修饰后类 t-石墨烯二维氮化硼的储氢性能改善
近年来,寻找具有高容量和优异H 2物理吸附性能的适用二维(2D)储氢材料引起了科学家和研究人员的广泛关注。根据合理的设计,并使用第一性原理计算,我们提出了一种用于储氢应用的t-石墨烯类氮化硼单层(tB 4 N 4 ),将t-石墨烯中的C原子替换为B和N原子。使用从头算分子动力学 (AIMD) 计算在 300 K 下评估了 tB 4 N 4系统上八边形中心吸附的锂原子的热稳定性和极化机制。此外,锂装饰的双面 tB4 N 4可以储存多达32个H 2分子,平均氢吸附能为0.217 eV/H 2,最大储氢容量为12.47 wt%。检查了吸附氢的可逆性,计算出的解吸温度为 161 K,远高于氢的临界点。基于扩散势垒, tB 4 N 4表面的H 2分子扩散动力学比t-石墨烯和石墨烯上的快。



























 京公网安备 11010802027423号
京公网安备 11010802027423号