当前位置:
X-MOL 学术
›
Polym. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and derivatization of epoxy-functional sterically-stabilized diblock copolymer spheres in non-polar media: does the spatial location of the epoxy groups matter?
Polymer Chemistry ( IF 4.6 ) Pub Date : 2022-06-10 , DOI: 10.1039/d2py00559j Csilla György 1 , Timothy Smith 2 , David J. Growney 2 , Steven P. Armes 1
Polymer Chemistry ( IF 4.6 ) Pub Date : 2022-06-10 , DOI: 10.1039/d2py00559j Csilla György 1 , Timothy Smith 2 , David J. Growney 2 , Steven P. Armes 1
Affiliation
|
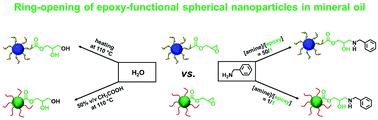
Epoxy-functional sterically-stabilized diblock copolymer spherical nanoparticles were synthesized via polymerization-induced self-assembly (PISA) in mineral oil. Epoxy groups were located either (i) in the nanoparticle cores or (ii) within the steric stabilizer chains. For the first system, reversible addition–fragmentation chain transfer (RAFT) dispersion polymerization of glycidyl methacrylate (GlyMA) was conducted using a poly(lauryl methacrylate) (PLMA63) precursor. The second system involved statistical copolymerization of GlyMA with lauryl methacrylate to produce a P(LMA50-stat-GlyMA9) precursor followed by chain extension using methyl methacrylate (MMA). 1H NMR studies and THF GPC analysis indicated that high monomer conversions (≥ 95%) and narrow molecular weight distributions (Mw/Mn ≤ 1.17) were obtained for both formulations. Dynamic light scattering indicated hydrodynamic diameters of 26 nm and 28 nm for P(LMA50-stat-GlyMA9)-PMMA67 and PLMA63-PGlyMA89 spheres, respectively. Transmission electron microscopy studies confirmed a well-defined spherical morphology in each case. Post-polymerization modification of these spherical nanoparticles was examined by reacting the epoxy groups with benzylamine. For the PLMA63-PGlyMA89 spheres, an [amine]/[epoxy] molar ratio of unity was sufficient to react all the epoxy groups. In contrast, the P(LMA50-stat-GlyMA9)-PMMA67 spheres required a fifty-fold excess of benzylamine for complete reaction. Furthermore, epoxy ring-opening reactions were conducted using either a trace amount of water or 50% v/v aqueous acetic acid at 110 °C. The extent of reaction was assessed using 1H NMR spectroscopy and THF GPC for the P(LMA50-stat-GlyMA9)-PMMA67 spheres and by FT-IR spectroscopy for the core-crosslinked PLMA63-PGlyMA89 spheres.
中文翻译:

非极性介质中环氧官能空间稳定二嵌段共聚物球体的合成和衍生:环氧基团的空间位置是否重要?
通过矿物油中的聚合诱导自组装 (PISA)合成了环氧功能空间稳定的二嵌段共聚物球形纳米颗粒。环氧基团位于(i)纳米颗粒核心或(ii)空间稳定剂链中。对于第一个系统,使用聚(甲基丙烯酸月桂酯)(PLMA 63)前体进行甲基丙烯酸缩水甘油酯(GlyMA)的可逆加成-断裂链转移(RAFT)分散聚合。第二种系统涉及 GlyMA 与甲基丙烯酸月桂酯的统计共聚以产生 P(LMA 50 - stat -GlyMA 9 ) 前体,然后使用甲基丙烯酸甲酯 (MMA) 进行链延长。1H NMR 研究和 THF GPC 分析表明,两种配方都获得了高单体转化率 (≥ 95%) 和窄分子量分布 ( M w / M n ≤ 1.17)。动态光散射表明 P(LMA 50 - stat -GlyMA 9 )-PMMA 67和 PLMA 63 -PGlyMA 89球体的流体动力学直径分别为 26 nm 和 28 nm 。透射电子显微镜研究证实在每种情况下都有明确的球形形态。通过使环氧基团与苄胺反应来检查这些球形纳米粒子的聚合后改性。对于 PLMA 63 -PGlyMA89个球体,[胺]/[环氧]摩尔比的统一足以使所有环氧基团反应。相比之下,P(LMA 50 - stat -GlyMA 9 )-PMMA 67球体需要 50 倍过量的苄胺才能完全反应。此外,在 110 °C 下使用痕量水或 50% v/v 乙酸水溶液进行环氧开环反应。使用1 H NMR 光谱和 THF GPC 评估 P(LMA 50 - stat -GlyMA 9 )-PMMA 67球体和通过 FT-IR 光谱学评估核心交联 PLMA 63 -PGlyMA 89球体的反应程度。
更新日期:2022-06-10
中文翻译:

非极性介质中环氧官能空间稳定二嵌段共聚物球体的合成和衍生:环氧基团的空间位置是否重要?
通过矿物油中的聚合诱导自组装 (PISA)合成了环氧功能空间稳定的二嵌段共聚物球形纳米颗粒。环氧基团位于(i)纳米颗粒核心或(ii)空间稳定剂链中。对于第一个系统,使用聚(甲基丙烯酸月桂酯)(PLMA 63)前体进行甲基丙烯酸缩水甘油酯(GlyMA)的可逆加成-断裂链转移(RAFT)分散聚合。第二种系统涉及 GlyMA 与甲基丙烯酸月桂酯的统计共聚以产生 P(LMA 50 - stat -GlyMA 9 ) 前体,然后使用甲基丙烯酸甲酯 (MMA) 进行链延长。1H NMR 研究和 THF GPC 分析表明,两种配方都获得了高单体转化率 (≥ 95%) 和窄分子量分布 ( M w / M n ≤ 1.17)。动态光散射表明 P(LMA 50 - stat -GlyMA 9 )-PMMA 67和 PLMA 63 -PGlyMA 89球体的流体动力学直径分别为 26 nm 和 28 nm 。透射电子显微镜研究证实在每种情况下都有明确的球形形态。通过使环氧基团与苄胺反应来检查这些球形纳米粒子的聚合后改性。对于 PLMA 63 -PGlyMA89个球体,[胺]/[环氧]摩尔比的统一足以使所有环氧基团反应。相比之下,P(LMA 50 - stat -GlyMA 9 )-PMMA 67球体需要 50 倍过量的苄胺才能完全反应。此外,在 110 °C 下使用痕量水或 50% v/v 乙酸水溶液进行环氧开环反应。使用1 H NMR 光谱和 THF GPC 评估 P(LMA 50 - stat -GlyMA 9 )-PMMA 67球体和通过 FT-IR 光谱学评估核心交联 PLMA 63 -PGlyMA 89球体的反应程度。


























 京公网安备 11010802027423号
京公网安备 11010802027423号