当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A nickel(II)-catalyzed enantioselective all-carbon-based inverse-electron-demand Diels–Alder reaction of 2-pyrones with indenes
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-06-10 , DOI: 10.1039/d2qo00493c Fangqing Zhang 1, 2 , Bing-Tao Ren 2 , Yangbin Liu 2 , Xiaoming Feng 2, 3
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-06-10 , DOI: 10.1039/d2qo00493c Fangqing Zhang 1, 2 , Bing-Tao Ren 2 , Yangbin Liu 2 , Xiaoming Feng 2, 3
Affiliation
|
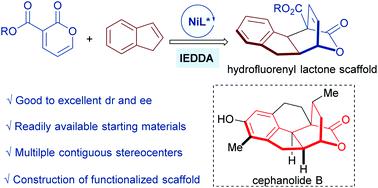
The Cephalotaxus norditerpenoids cephanolides A–D feature a densely functionalized hexahydrofluorenyl bridged-lactone scaffold. To concisely achieve these privileged structures, herein, an asymmetric inverse-electron-demand Diels–Alder (IEDDA) reaction of electron-deficient 2-pyrones with electronically unbiased indenes catalyzed by a chiral N,N′-dioxide/Ni(OTf)2 complex has been disclosed. Based on this reaction, a variety of substituted hexahydrofluorenyl lactone scaffolds were obtained with good to excellent yields (up to 98% yield) and enantioselectivities (up to 93% ee) under mild conditions.
中文翻译:

2-吡喃酮与茚的镍(II)催化对映选择性全碳基逆电子需求Diels-Alder反应
Cephalotaxus norditerpenoids cephanolides A-D 具有密集功能化的六氢芴基桥连内酯支架。为了简洁地实现这些特权结构,本文中,缺电子的 2-吡喃酮与电子无偏茚在手性N , N '-二氧化物/Ni(OTf) 2催化下的不对称逆电子需求 Diels-Alder (IEDDA) 反应复杂的已被披露。基于该反应,在温和条件下获得了多种取代的六氢芴基内酯支架,其产率良好(高达 98%)和对映选择性(高达 93% ee)。
更新日期:2022-06-10
中文翻译:

2-吡喃酮与茚的镍(II)催化对映选择性全碳基逆电子需求Diels-Alder反应
Cephalotaxus norditerpenoids cephanolides A-D 具有密集功能化的六氢芴基桥连内酯支架。为了简洁地实现这些特权结构,本文中,缺电子的 2-吡喃酮与电子无偏茚在手性N , N '-二氧化物/Ni(OTf) 2催化下的不对称逆电子需求 Diels-Alder (IEDDA) 反应复杂的已被披露。基于该反应,在温和条件下获得了多种取代的六氢芴基内酯支架,其产率良好(高达 98%)和对映选择性(高达 93% ee)。


























 京公网安备 11010802027423号
京公网安备 11010802027423号