当前位置:
X-MOL 学术
›
Adv. Therap.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tumor Microenvironment-Activated Nanosystem With High Aggregation and On-Demand Degradation for Imaging-Guided Synergistic Hydrogenothermal Therapy
Advanced Therapeutics ( IF 4.6 ) Pub Date : 2022-06-09 , DOI: 10.1002/adtp.202200056 Yufei Qin 1 , Ziliang Zheng 1 , Xuejiao Chen 1 , Qin Liu 2 , Shilei Ren 3 , Weiwei Zhang 2 , Ailin Duan 1 , Ruiping Zhang 2
Advanced Therapeutics ( IF 4.6 ) Pub Date : 2022-06-09 , DOI: 10.1002/adtp.202200056 Yufei Qin 1 , Ziliang Zheng 1 , Xuejiao Chen 1 , Qin Liu 2 , Shilei Ren 3 , Weiwei Zhang 2 , Ailin Duan 1 , Ruiping Zhang 2
Affiliation
|
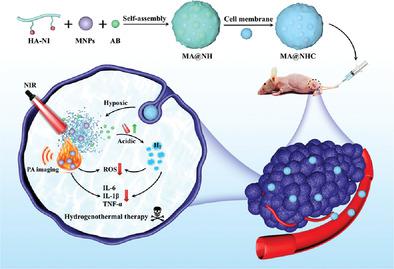
Photothermal therapy (PTT) as an emerging antitumor approach has many advantages. However, when the PTT generates localized hyperthermia to induce cell apoptosis, excess reactive oxygen species (ROS) and inflammatory cytokine release will threaten the peritumoral healthy tissues, thus it is challenging to develop efficient strategies to reduce the undesirable PTT-mediated side effects. Herein, the authors report exploitation of hypoxia-responsive degradation in designing a synergistic hydrogenothermal therapeutic nanosystem for ROS elimination and anti-inflammatory action to enhance antitumor activity. The nanosystem (MA@NHC) is successfully constructed by encapsulating melanin-nanoparticles (MNPs) and amine-borane (AB) inside functionalized hyaluronic acid to obtain MA@NH, then capped with 4T1 cell-membranes. Synergistic integration of the homotypic aggregation with EPR effect can improve the tumor-targeting properties of MA@NHC. Upon specifically targeting tumor, sequentially time-dependent MNPs and AB release can be triggered by hypoxia tumor-microenvironment. Based on the superior NIR light-absorption ability, widely permeated MNPs are activated for photoacoustic imaging-guided PTT. Meanwhile, the high intra-tumor acidity accelerates the production of H2 via AB to scavenge excess ROS and reduce PTT-induced inflammatory responses, which can be further sped up during PTT. Remarkably, as an antioxidant and anti-inflammatory agent, the MNPs synergize with H2 to protect peritumoral normal cells from damage.
中文翻译:

具有高聚集和按需降解的肿瘤微环境激活纳米系统用于成像引导的协同水热疗法
光热疗法(PTT)作为一种新兴的抗肿瘤方法具有许多优点。然而,当 PTT 产生局部热疗以诱导细胞凋亡时,过量的活性氧 (ROS) 和炎性细胞因子的释放将威胁到肿瘤周围的健康组织,因此制定有效的策略来减少不良 PTT 介导的副作用具有挑战性。在此,作者报告了利用缺氧响应降解设计协同氢热治疗纳米系统,以消除 ROS 和抗炎作用以增强抗肿瘤活性。通过将黑色素纳米颗粒(MNPs)和胺硼烷(AB)包裹在功能化透明质酸中以获得MA@NH,然后用4T1细胞膜覆盖,成功构建了纳米系统(MA@NHC)。同型聚集与 EPR 效应的协同整合可以提高 MA@NHC 的肿瘤靶向特性。在特异性靶向肿瘤后,缺氧肿瘤微环境可以触发顺序时间依赖性的 MNP 和 AB 释放。基于优越的近红外光吸收能力,广泛渗透的 MNP 被激活用于光声成像引导的 PTT。同时,高肿瘤内酸度加速了H的产生2通过 AB 清除多余的 ROS 并减少 PTT 诱导的炎症反应,在 PTT 期间可以进一步加速。值得注意的是,作为抗氧化剂和抗炎剂,MNPs 与 H 2协同作用以保护瘤周正常细胞免受损伤。
更新日期:2022-06-09
中文翻译:

具有高聚集和按需降解的肿瘤微环境激活纳米系统用于成像引导的协同水热疗法
光热疗法(PTT)作为一种新兴的抗肿瘤方法具有许多优点。然而,当 PTT 产生局部热疗以诱导细胞凋亡时,过量的活性氧 (ROS) 和炎性细胞因子的释放将威胁到肿瘤周围的健康组织,因此制定有效的策略来减少不良 PTT 介导的副作用具有挑战性。在此,作者报告了利用缺氧响应降解设计协同氢热治疗纳米系统,以消除 ROS 和抗炎作用以增强抗肿瘤活性。通过将黑色素纳米颗粒(MNPs)和胺硼烷(AB)包裹在功能化透明质酸中以获得MA@NH,然后用4T1细胞膜覆盖,成功构建了纳米系统(MA@NHC)。同型聚集与 EPR 效应的协同整合可以提高 MA@NHC 的肿瘤靶向特性。在特异性靶向肿瘤后,缺氧肿瘤微环境可以触发顺序时间依赖性的 MNP 和 AB 释放。基于优越的近红外光吸收能力,广泛渗透的 MNP 被激活用于光声成像引导的 PTT。同时,高肿瘤内酸度加速了H的产生2通过 AB 清除多余的 ROS 并减少 PTT 诱导的炎症反应,在 PTT 期间可以进一步加速。值得注意的是,作为抗氧化剂和抗炎剂,MNPs 与 H 2协同作用以保护瘤周正常细胞免受损伤。



























 京公网安备 11010802027423号
京公网安备 11010802027423号