Electrochimica Acta ( IF 6.6 ) Pub Date : 2022-06-09 , DOI: 10.1016/j.electacta.2022.140696 Yoganandan Govindaraj , Durgambika Venkatachalam , Manoj Prabhakar , Natarajan Thayee Manikandanath , Jayam Nagabushan Balaraju , Michael Rohwerder , Lakshman Neelakantan
|
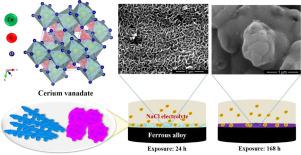
The synthesized, nano-sized cerium vanadate is proposed as a self-healing corrosion inhibitor for ferrous alloys (e.g., automotive high strength steel (HSS) and mild steel (MS)). Cerium vanadate prepared at two different pH conditions (neutral and basic) showed similar corn-like morphology with nanorod structure. UV-Vis spectroscopy studies revealed that the release rate of cerium vanadate-B (basic condition) was higher than cerium vanadate-N (neutral condition) in 0.1 M NaCl solution. The specimen exposed to 0.1 M NaCl containing a supersaturated solution of cerium vanadate-B (1000 ppm) revealed 8 times and 6 times lower corrosion current density values for HSS and MS respectively than that of the one without corrosion inhibitor. There was a gradual increase in the film resistance (Rfilm) on both HSS and MS observed as a function of exposure time in corrosive medium containing cerium vanadate-B inhibitor. An order higher impedance values were observed for both HSS and MS immersed for 168 h, highlighting the self-healing effect of the cerium vanadate compound. The X-ray photoelectron spectroscopy results showed the presence of multivalent oxidation states of both cerium and vanadium species. The inhibiting action is attributed to the high solubility of cerium vanadate in the corrosive medium to form a film on the metal surface. The dissolution/solubility of the corrosion inhibitor was more favorable in neutral to alkaline conditions than in acidic conditions.
中文翻译:

纳米铈钒氧化物作为缓蚀剂:微观结构和释放研究
合成的纳米尺寸钒酸铈被提议作为铁合金(例如,汽车高强度钢 (HSS) 和低碳钢 (MS))的自愈缓蚀剂。在两种不同的 pH 条件(中性和碱性)下制备的钒酸铈显示出类似的具有纳米棒结构的玉米状形态。UV-Vis光谱研究表明,钒酸铈-B(碱性条件)在0.1 M NaCl溶液中的释放速率高于钒酸铈-N(中性条件)。暴露于含有钒酸铈-B (1000 ppm) 过饱和溶液的 0.1 M NaCl 中的样品显示,HSS 和 MS 的腐蚀电流密度值分别比不含缓蚀剂的样品低 8 倍和 6 倍。膜电阻逐渐增加(R膜) 在 HSS 和 MS 上观察到作为在含有钒酸铈-B 抑制剂的腐蚀性介质中暴露时间的函数。浸泡 168 小时的 HSS 和 MS 均观察到更高的阻抗值,突出了钒酸铈化合物的自愈效果。X 射线光电子能谱结果表明铈和钒物质都存在多价氧化态。抑制作用归因于钒酸铈在腐蚀介质中的高溶解度,在金属表面形成一层薄膜。缓蚀剂的溶解/溶解性在中性至碱性条件下比在酸性条件下更有利。


























 京公网安备 11010802027423号
京公网安备 11010802027423号