Cell ( IF 64.5 ) Pub Date : 2022-06-09 , DOI: 10.1016/j.cell.2022.05.008 Erinc Hallacli 1 , Can Kayatekin 2 , Sumaiya Nazeen 3 , Xiou H Wang 4 , Zoe Sheinkopf 4 , Shubhangi Sathyakumar 4 , Souvarish Sarkar 5 , Xin Jiang 6 , Xianjun Dong 7 , Roberto Di Maio 8 , Wen Wang 9 , Matthew T Keeney 8 , Daniel Felsky 10 , Jackson Sandoe 2 , Aazam Vahdatshoar 4 , Namrata D Udeshi 11 , D R Mani 11 , Steven A Carr 11 , Susan Lindquist 12 , Philip L De Jager 13 , David P Bartel 12 , Chad L Myers 9 , J Timothy Greenamyre 8 , Mel B Feany 5 , Shamil R Sunyaev 14 , Chee Yeun Chung 6 , Vikram Khurana 15
|
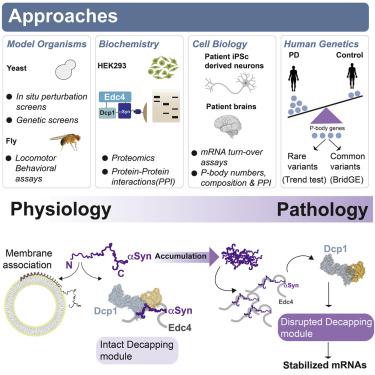
Alpha-synuclein (αS) is a conformationally plastic protein that reversibly binds to cellular membranes. It aggregates and is genetically linked to Parkinson’s disease (PD). Here, we show that αS directly modulates processing bodies (P-bodies), membraneless organelles that function in mRNA turnover and storage. The N terminus of αS, but not other synucleins, dictates mutually exclusive binding either to cellular membranes or to P-bodies in the cytosol. αS associates with multiple decapping proteins in close proximity on the Edc4 scaffold. As αS pathologically accumulates, aberrant interaction with Edc4 occurs at the expense of physiologic decapping-module interactions. mRNA decay kinetics within PD-relevant pathways are correspondingly disrupted in PD patient neurons and brain. Genetic modulation of P-body components alters αS toxicity, and human genetic analysis lends support to the disease-relevance of these interactions. Beyond revealing an unexpected aspect of αS function and pathology, our data highlight the versatility of conformationally plastic proteins with high intrinsic disorder.
中文翻译:

帕金森病蛋白 α-突触核蛋白是加工体和 mRNA 稳定性的调节剂
α-突触核蛋白 (αS) 是一种构象可塑性蛋白,可可逆地与细胞膜结合。它聚集并在遗传上与帕金森病 (PD) 相关。在这里,我们表明 αS 直接调节处理体(P-体),即在 mRNA 周转和储存中起作用的无膜细胞器。αS 的 N 末端,而不是其他突触核蛋白,决定了与细胞膜或胞质溶胶中的 P 体相互排斥的结合。αS 与 Edc4 支架上的多个脱帽蛋白结合。随着 αS 的病理积累,与 Edc4 的异常相互作用是以生理去盖模块相互作用为代价的。PD 相关通路中的 mRNA 衰变动力学在 PD 患者的神经元和大脑中相应地被破坏。P体成分的遗传调节改变了αS毒性,和人类遗传分析为这些相互作用的疾病相关性提供了支持。除了揭示 αS 功能和病理学的一个意想不到的方面外,我们的数据还突出了具有高度内在紊乱的构象可塑性蛋白的多功能性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号