当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Accelerating the dissolution kinetics of iodine with a cosolvent for a high-current zinc–iodine flow battery
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2022-06-09 , DOI: 10.1039/d2ta03195g Yunhe Zhao 1 , Yang Li 1 , Jiatao Mao 2 , Zhibin Yi 1 , Nauman Mubarak 1 , Yiting Zheng 1 , Jang-Kyo Kim 1 , Qing Chen 1, 2, 3
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2022-06-09 , DOI: 10.1039/d2ta03195g Yunhe Zhao 1 , Yang Li 1 , Jiatao Mao 2 , Zhibin Yi 1 , Nauman Mubarak 1 , Yiting Zheng 1 , Jang-Kyo Kim 1 , Qing Chen 1, 2, 3
Affiliation
|
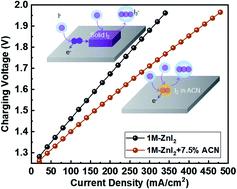
The high reduction potential and the abundance of iodine have prompted its use in the positive electrolytes of aqueous flow batteries, where the transition between highly soluble iodide (I−) and triiodide (I3−) may give rise to superior rate-performance. Yet, the operating currents of Zn–I2 flow batteries remain low, for which the elusive kinetics of the poorly soluble intermediate, iodine (I2), is responsible. With cyclic voltammetry and chronoamperometry, we confirm that solid iodine passivates the carbon electrode, leading to an oscillating current even in a highly concentrated I− solution. UV-Vis spectroscopy and an interface-controlled dissolution model are used to measure the rate constant of the transition from I2 to I3− to be ∼10−6 mol (cm−2 s−1), which agrees with the steady-state current revealed by chronoamperometry and corroborates the current-limiting kinetics. To boost the kinetics, we screen common organic solvents for a cosolvent that can better solvate I2 and find that the addition of 7.5 vol% acetonitrile increases the rate constant by an order of magnitude and the current by more than six times. Calculations using density functional theory reveal the role of the cosolvent in weakening the interaction between iodine and the electrode surface. When employed in a Zn–I2 flow battery, the cosolvent enables a high charging current of over 100 mA cm−2, which is stable for more than 170 cycles, and is promising for high-performance energy storage at an affordable cost.
中文翻译:

用助溶剂加速碘的溶解动力学,用于大电流锌碘液流电池
高还原电位和丰富的碘促使其在水系液流电池的正极电解质中得到应用,其中高可溶性碘化物 (I - ) 和三碘化物 (I 3 - ) 之间的转变可能会产生优异的倍率性能。然而,Zn-I 2液流电池的工作电流仍然很低,这与难溶性中间体碘 (I 2 ) 难以捉摸的动力学有关。通过循环伏安法和计时电流法,我们确认固体碘钝化碳电极,即使在高度集中的 I 中也会产生振荡电流-解决方案。UV-Vis 光谱和界面控制的溶解模型用于测量从 I 2到 I 3 -转变的速率常数为∼10 -6 mol (cm -2 s -1 ),这与稳态一致计时电流法揭示的状态电流并证实了限流动力学。为了提高动力学,我们筛选了常见的有机溶剂,寻找可以更好地溶剂化 I 2的助溶剂并发现添加 7.5 vol% 的乙腈使速率常数增加了一个数量级,电流增加了 6 倍以上。使用密度泛函理论的计算揭示了助溶剂在削弱碘和电极表面之间的相互作用中的作用。当用于 Zn-I 2液流电池时,该助溶剂可实现超过 100 mA cm -2的高充电电流,可稳定循环超过 170 次,有望以可承受的成本实现高性能储能。
更新日期:2022-06-09
中文翻译:

用助溶剂加速碘的溶解动力学,用于大电流锌碘液流电池
高还原电位和丰富的碘促使其在水系液流电池的正极电解质中得到应用,其中高可溶性碘化物 (I - ) 和三碘化物 (I 3 - ) 之间的转变可能会产生优异的倍率性能。然而,Zn-I 2液流电池的工作电流仍然很低,这与难溶性中间体碘 (I 2 ) 难以捉摸的动力学有关。通过循环伏安法和计时电流法,我们确认固体碘钝化碳电极,即使在高度集中的 I 中也会产生振荡电流-解决方案。UV-Vis 光谱和界面控制的溶解模型用于测量从 I 2到 I 3 -转变的速率常数为∼10 -6 mol (cm -2 s -1 ),这与稳态一致计时电流法揭示的状态电流并证实了限流动力学。为了提高动力学,我们筛选了常见的有机溶剂,寻找可以更好地溶剂化 I 2的助溶剂并发现添加 7.5 vol% 的乙腈使速率常数增加了一个数量级,电流增加了 6 倍以上。使用密度泛函理论的计算揭示了助溶剂在削弱碘和电极表面之间的相互作用中的作用。当用于 Zn-I 2液流电池时,该助溶剂可实现超过 100 mA cm -2的高充电电流,可稳定循环超过 170 次,有望以可承受的成本实现高性能储能。



























 京公网安备 11010802027423号
京公网安备 11010802027423号