当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Theoretical study on Y-doped Na2ZrO3 as a high-capacity Na-rich cathode material based on anionic redox
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2022-06-09 , DOI: 10.1039/d2cp02219b Huimin Yin 1 , Jiajia Huang 1 , Ningjing Luo 1 , Yongfan Zhang 1 , Shuping Huang 1, 2
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2022-06-09 , DOI: 10.1039/d2cp02219b Huimin Yin 1 , Jiajia Huang 1 , Ningjing Luo 1 , Yongfan Zhang 1 , Shuping Huang 1, 2
Affiliation
|
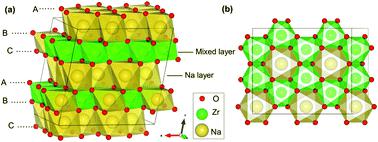
First-principles calculations based on density functional theory were utilized to study the performance of Na2ZrO3 (NZO) and yttrium-doped Na2ZrO3 (Y-NZO) as cathode materials for sodium ion batteries (SIBs), including the stability of the desodiated structures, desodiation energy, redox mechanism, and the diffusion of Na. When 62.5% sodium is removed from NZO, its structure and volume change little and the layered structure is retained, whereas the structure starts to distort and shift to the ZrO3 phase with the extraction of more than 62.5% sodium. As desodiation proceeds, oxygen anions act as the only redox center for charge compensation, yielding a high initial voltage of 4.03 eV vs. Na/Na+ by PBE + U-D3 functional and 4.82 eV vs. Na/Na+ by HSE06-D3 functional. When the desodiation content is less than 31.25%, O23− is formed with an O–O distance of 2.38 Å. At the desodiation content of 31.25%, peroxide dimer O22− starts to form; at the desodiation content of 56.25%, the O–O bond distance is further shortened to 1.3 Å, corresponding to the formation of superoxide O2−. However, for Y-NZO, the redox reaction firstly involves O2−/O1−, which does not occur in NZO. Peroxides and superoxides appear when the sodium removal concentration is 59.38% and 75%, respectively. This indicates that the O–O dimers appear in Y-NZO at much deeper sodium removal. The calculations of diffusion paths and barriers of Na ions in NZO by PBE + U-D3 predict that the barrier of Na escaping from the mixed layer to the Na layer in NZO is 0.48 eV (the reverse barrier is 0.76 eV), smaller than those of other O3-type layered transition metal compounds, such as Na2IrO3 and Na2RuO3. After yttrium doping, the diffusion of Na ions becomes easier, indicating that the Y-doping improves the diffusion ability. This investigation interprets the mechanism of oxygen oxidation of NZO as a cathode for SIBs, and provides theoretical support for a better design of Na-rich layered oxide Na2MO3 (M represents the transition metal element) in the future research.
中文翻译:

Y掺杂Na2ZrO3作为基于阴离子氧化还原的高容量富钠正极材料的理论研究
利用基于密度泛函理论的第一性原理计算研究了Na 2 ZrO 3 (NZO)和钇掺杂的Na 2 ZrO 3 (Y-NZO)作为钠离子电池(SIBs)正极材料的性能,包括稳定性脱钠结构、脱钠能、氧化还原机理和钠的扩散。当从NZO中脱除62.5%的钠时,其结构和体积变化不大,层状结构得以保留,而当钠的提取量超过62.5%时,结构开始变形并转变为ZrO 3相。随着脱钠过程的进行,氧阴离子充当电荷补偿的唯一氧化还原中心,产生 4.03 eV 的高初始电压(相对于 4.03 eV)。Na/Na +由 PBE + U -D3 功能和 4.82 eV vs. Na/Na +通过 HSE06-D3 功能。当脱钠含量小于 31.25% 时,O 2 3-形成,O-O 距离为 2.38 Å。在脱钠含量为 31.25% 时,开始形成过氧化物二聚体 O 2 2− ;在脱钠含量为56.25%时,O-O键距离进一步缩短至1.3 Å,对应于超氧化物O 2 -的形成。然而,对于 Y-NZO,氧化还原反应首先涉及 O 2- /O 1-,这在 NZO 中不会发生。脱钠浓度分别为59.38%和75%时出现过氧化物和超氧化物。这表明 O-O 二聚体出现在 Y-NZO 中以更深的钠去除。通过 PBE + U -D3 计算 Na 离子在 NZO 中的扩散路径和势垒,预测 Na 从混合层逃逸到 NZO 中的 Na 层的势垒为 0.48 eV(反向势垒为 0.76 eV),小于那些其他 O3 型层状过渡金属化合物,如 Na 2 IrO 3和 Na 2 RuO 3. 钇掺杂后,Na离子的扩散变得更容易,表明Y掺杂提高了扩散能力。本研究解释了NZO作为SIBs正极的氧氧化机理,为未来研究中更好地设计富钠层状氧化物Na 2 MO 3(M代表过渡金属元素)提供了理论支持。
更新日期:2022-06-09
中文翻译:

Y掺杂Na2ZrO3作为基于阴离子氧化还原的高容量富钠正极材料的理论研究
利用基于密度泛函理论的第一性原理计算研究了Na 2 ZrO 3 (NZO)和钇掺杂的Na 2 ZrO 3 (Y-NZO)作为钠离子电池(SIBs)正极材料的性能,包括稳定性脱钠结构、脱钠能、氧化还原机理和钠的扩散。当从NZO中脱除62.5%的钠时,其结构和体积变化不大,层状结构得以保留,而当钠的提取量超过62.5%时,结构开始变形并转变为ZrO 3相。随着脱钠过程的进行,氧阴离子充当电荷补偿的唯一氧化还原中心,产生 4.03 eV 的高初始电压(相对于 4.03 eV)。Na/Na +由 PBE + U -D3 功能和 4.82 eV vs. Na/Na +通过 HSE06-D3 功能。当脱钠含量小于 31.25% 时,O 2 3-形成,O-O 距离为 2.38 Å。在脱钠含量为 31.25% 时,开始形成过氧化物二聚体 O 2 2− ;在脱钠含量为56.25%时,O-O键距离进一步缩短至1.3 Å,对应于超氧化物O 2 -的形成。然而,对于 Y-NZO,氧化还原反应首先涉及 O 2- /O 1-,这在 NZO 中不会发生。脱钠浓度分别为59.38%和75%时出现过氧化物和超氧化物。这表明 O-O 二聚体出现在 Y-NZO 中以更深的钠去除。通过 PBE + U -D3 计算 Na 离子在 NZO 中的扩散路径和势垒,预测 Na 从混合层逃逸到 NZO 中的 Na 层的势垒为 0.48 eV(反向势垒为 0.76 eV),小于那些其他 O3 型层状过渡金属化合物,如 Na 2 IrO 3和 Na 2 RuO 3. 钇掺杂后,Na离子的扩散变得更容易,表明Y掺杂提高了扩散能力。本研究解释了NZO作为SIBs正极的氧氧化机理,为未来研究中更好地设计富钠层状氧化物Na 2 MO 3(M代表过渡金属元素)提供了理论支持。



























 京公网安备 11010802027423号
京公网安备 11010802027423号