当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photochemically triggered cheletropic formation of cyclopropenone (c-C3H2O) from carbon monoxide and electronically excited acetylene
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2022-06-09 , DOI: 10.1039/d2cp01978g Jia Wang 1, 2 , N Fabian Kleimeier 1, 2 , Rebecca N Johnson 3 , Samer Gozem 3 , Matthew J Abplanalp 1, 2 , Andrew M Turner 1, 2 , Joshua H Marks 1, 2 , Ralf I Kaiser 1, 2
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2022-06-09 , DOI: 10.1039/d2cp01978g Jia Wang 1, 2 , N Fabian Kleimeier 1, 2 , Rebecca N Johnson 3 , Samer Gozem 3 , Matthew J Abplanalp 1, 2 , Andrew M Turner 1, 2 , Joshua H Marks 1, 2 , Ralf I Kaiser 1, 2
Affiliation
|
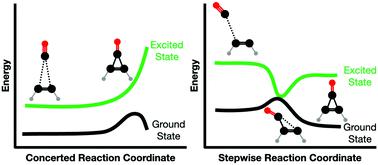
For more than half a century, pericyclic reactions have played an important role in advancing our fundamental understanding of cycloadditions, sigmatropic shifts, group transfer reactions, and electrocyclization reactions. However, the fundamental mechanisms of photochemically activated cheletropic reactions have remained contentious. Here we report on the simplest cheletropic reaction: the [2+1] addition of ground state 18O-carbon monoxide (C18O, X1Σ+) to D2-acetylene (C2D2) photochemically excited to the first excited triplet (T1), second excited triplet (T2), and first excited singlet state (S1) at 5 K, leading to the formation of D2-18O-cyclopropenone (c-C3D218O). Supported by quantum-chemical calculations, our investigation provides persuasive testimony on stepwise cheletropic reaction pathways to cyclopropenone via excited state dynamics involving the T2 (non-adiabatic) and S1 state (adiabatic) of acetylene at 5 K, while the T1 state energetically favors an intermediate structure that directly dissociates after relaxing to the ground state. The agreement between experiments in low temperature ices and the excited state calculations signifies how photolysis experiments coupled with theoretical calculations can untangle polyatomic reactions with relevance to fundamental physical organic chemistry at the molecular level, thus affording a versatile strategy to unravel exotic non-equilibrium chemistries in cyclic, aromatic organics. Distinct from traditional radical–radical pathways leading to organic molecules on ice-coated interstellar nanoparticles (interstellar grains) in cold molecular clouds and star-forming regions, the photolytic formation of cyclopropenone as presented changes the perception of how we explain the formation of complex organics in the interstellar medium eventually leading to the molecular precursors of biorelevant molecules.
中文翻译:

从一氧化碳和电子激发的乙炔光化学引发的螯合形成环丙烯酮 (c-C3H2O)
半个多世纪以来,周环反应在促进我们对环加成、σ 位移、基团转移反应和电环化反应的基本理解方面发挥了重要作用。然而,光化学活化的螯合反应的基本机制仍然存在争议。在这里,我们报告了最简单的螯合反应:将基态18 O-一氧化碳 (C 18 O, X 1 Σ + ) 添加到 D2-乙炔 (C 2 D 2 ) 中的 [2+1] 光化学激发到第一个激发三重态 (T1)、第二个激发三重态 (T2) 和第一个激发单重态 (S1) 在 5 K,导致 D2-18 的形成O-环丙烯酮 (cC 3 D 2 18 O)。在量子化学计算的支持下,我们的研究为环丙烯酮的逐步螯合反应途径提供了有说服力的证据。激发态动力学涉及乙炔在 5 K 时的 T2(非绝热)和 S1 态(绝热),而 T1 态在能量上倾向于中间结构,该中间结构在松弛至基态后直接解离。低温冰实验与激发态计算之间的一致性表明,光解实验与理论计算相结合,可以在分子水平上解开与基本物理有机化学相关的多原子反应,从而为解开奇异的非平衡化学提供了一种通用策略。环状、芳香族有机物。不同于在冷分子云和恒星形成区域中导致冰覆盖的星际纳米粒子(星际颗粒)上的有机分子的传统自由基 - 自由基途径,
更新日期:2022-06-09
中文翻译:

从一氧化碳和电子激发的乙炔光化学引发的螯合形成环丙烯酮 (c-C3H2O)
半个多世纪以来,周环反应在促进我们对环加成、σ 位移、基团转移反应和电环化反应的基本理解方面发挥了重要作用。然而,光化学活化的螯合反应的基本机制仍然存在争议。在这里,我们报告了最简单的螯合反应:将基态18 O-一氧化碳 (C 18 O, X 1 Σ + ) 添加到 D2-乙炔 (C 2 D 2 ) 中的 [2+1] 光化学激发到第一个激发三重态 (T1)、第二个激发三重态 (T2) 和第一个激发单重态 (S1) 在 5 K,导致 D2-18 的形成O-环丙烯酮 (cC 3 D 2 18 O)。在量子化学计算的支持下,我们的研究为环丙烯酮的逐步螯合反应途径提供了有说服力的证据。激发态动力学涉及乙炔在 5 K 时的 T2(非绝热)和 S1 态(绝热),而 T1 态在能量上倾向于中间结构,该中间结构在松弛至基态后直接解离。低温冰实验与激发态计算之间的一致性表明,光解实验与理论计算相结合,可以在分子水平上解开与基本物理有机化学相关的多原子反应,从而为解开奇异的非平衡化学提供了一种通用策略。环状、芳香族有机物。不同于在冷分子云和恒星形成区域中导致冰覆盖的星际纳米粒子(星际颗粒)上的有机分子的传统自由基 - 自由基途径,



























 京公网安备 11010802027423号
京公网安备 11010802027423号