Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reply to the ‘Comment on “Realization of the Zn3+ oxidation state”’ by Y. Shang, N. Shu, Z. Zhang, P. Yang and J. Xu, Nanoscale, 2022, 14, DOI: 10.1039/D1NR07031B
Nanoscale ( IF 6.7 ) Pub Date : 2022-06-09 , DOI: 10.1039/d2nr01066f H Fang 1 , H Banjade 1 , Deepika 1 , P Jena 1
Nanoscale ( IF 6.7 ) Pub Date : 2022-06-09 , DOI: 10.1039/d2nr01066f H Fang 1 , H Banjade 1 , Deepika 1 , P Jena 1
Affiliation
|
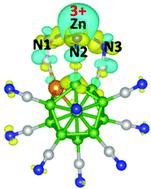
In a recent paper (https://doi.org/10.1039/D1NR02816B), we suggested that Zn can assume a +3-oxidation state when interacting with super-electrophilic clusters, BeB11(CN)12 and BeB23(CN)22. In a comment to our paper (https://doi.org/10.1039/D1NR07031B), Shang et al. have questioned this suggestion. Using density functional theory with the TPSSh functional and def2-SVP basis sets in the Gaussian16 software and semiempirical localized orbital bonding analysis (LOBA), the authors have made three major claims: (1) the oxidation state of Zn in Zn[BeB11(CN)12] and Zn[BeB23(CN)22] is +2; (2) electron affinities are not reliable to probe the oxidation states; and (3) our results are “misleading” because these are based on the VASP code. According to these authors, VASP is not suitable for small clusters because it uses projected augmented wave (PAW) pseudopotentials. In the following, we show that these claims are invalid, caused by both misunderstanding and the authors’ use of a lower-level theory.
更新日期:2022-06-09



























 京公网安备 11010802027423号
京公网安备 11010802027423号