Minerals Engineering ( IF 4.8 ) Pub Date : 2022-06-08 , DOI: 10.1016/j.mineng.2022.107630 Sana Zahid , Hans C. Oskierski , Ibukun Oluwoye , Helen E.A. Brand , Fang Xia , Gamini Senanayake , Mohammednoor Altarawneh , Bogdan Z. Dlugogorski
|
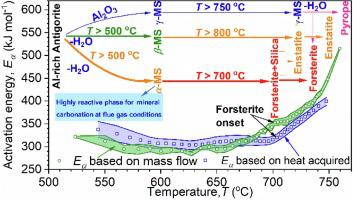
Heat-treatment of serpentine minerals generates structural amorphicity and increases reactivity during subsequent mineral carbonation, a strategy for large-scale sequestration of CO2. This study employs thermal analyses (TGA-DSC) in conjunction with in-situ synchrotron powder X-ray diffraction (PXRD) to record concurrent mass loss, heat flow, and mineralogical changes during thermal treatment of antigorite. Isoconversional kinetic modelling demonstrates that thermal decomposition of antigorite is a complex multi-step reaction, with activation energies (Eα) varying between 290 and 515 kJ mol−1. We identify three intermediate phases forming during antigorite dehydroxylation, a semi-crystalline chlorite-like phase (γ-metaserpentine) showing an additional reaction pathway for the decomposition of Al2O3-rich antigorite into pyrope, and two distinct amorphous components (α and β-metaserpentine) which convert into forsterite and enstatite at higher temperature, respectively. The combination of isoconversional kinetics with in-situ synchrotron PXRD illustrates, for the first time, that local crystal structure changes, related to intermediate phase and forsterite formation, are responsible for the steep increase in activation energy above 650 °C and only 49% dehydroxylation can be achieved prior to this increase. This suggests that the high thermal stability of Al2O3-rich antigorite would severely limit Mg extraction during application of mineral carbonation under flue gas conditions.
中文翻译:

用于二氧化碳封存的叶蛇纹石脱羟基动力学
蛇纹石矿物的热处理会产生结构非晶性并在随后的矿物碳化过程中增加反应性,这是一种大规模封存 CO 2的策略。本研究采用热分析 (TGA-DSC) 结合原位同步加速器粉末 X 射线衍射 (PXRD) 来记录叶蛇纹石热处理过程中同时发生的质量损失、热流和矿物学变化。等转化动力学模型表明叶蛇纹石的热分解是一个复杂的多步反应,活化能 ( E α ) 在 290 和 515 kJ mol -1之间变化。我们确定了在叶蛇纹石脱羟基过程中形成的三个中间相,一种半结晶的亚氯酸盐相(γ-偏蛇纹石)显示了将富含 Al 2 O 3的叶蛇纹石分解为镁铝榴石的附加反应途径,以及在较高温度下分别转化为镁橄榄石和顽火辉石的两种不同的无定形组分( α和β-偏蛇纹石)。等转化动力学与原位同步加速器 PXRD 的结合首次表明,与中间相和镁橄榄石形成相关的局部晶体结构变化是导致 650 °C 以上活化能急剧增加和仅 49% 脱羟基的原因可以在此增加之前实现。这表明 Al 2 O 3的高热稳定性富叶蛇纹石会严重限制在烟气条件下应用矿物碳化过程中镁的提取。


























 京公网安备 11010802027423号
京公网安备 11010802027423号