当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enantioselective iodolactonization to prepare ε-lactone rings using hypervalent iodine
Chemical Science ( IF 8.4 ) Pub Date : 2022-06-08 , DOI: 10.1039/d2sc01587k Jenna L Payne 1 , Zihang Deng 1 , Andrew L Flach 1 , Jeffrey N Johnston 1
Chemical Science ( IF 8.4 ) Pub Date : 2022-06-08 , DOI: 10.1039/d2sc01587k Jenna L Payne 1 , Zihang Deng 1 , Andrew L Flach 1 , Jeffrey N Johnston 1
Affiliation
|
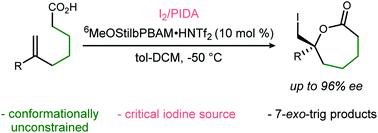
Despite the rapid growth of enantioselective halolactonization reactions in recent years, most are effective only when forming smaller (6,5,4-membered) rings. Seven-membered ε-lactones, are rarely formed with high selectivity, and never without conformational bias. We describe the first highly enantioselective 7-exo-trig iodolactonizations of conformationally unbiased ε-unsaturated carboxylic acids, effected by an unusual combination of a bifunctional BAM catalyst, I2, and I(III) reagent (PhI(OAc)2:PIDA).
中文翻译:

高价碘对映选择性碘内酯化制备 ε-内酯环
尽管近年来对映选择性卤内酯化反应迅速增长,但大多数仅在形成较小的(6,5,4-元)环时才有效。七元 ε-内酯很少以高选择性形成,而且从来没有没有构象偏差。我们描述了第一个高度对映选择性的 7 - exo -trig iodolactonizations 构象无偏的 ε-不饱和羧酸,由双功能 BAM 催化剂、I 2和 I( III ) 试剂 (PhI(OAc) 2 :PIDA)的不寻常组合影响.
更新日期:2022-06-08
中文翻译:

高价碘对映选择性碘内酯化制备 ε-内酯环
尽管近年来对映选择性卤内酯化反应迅速增长,但大多数仅在形成较小的(6,5,4-元)环时才有效。七元 ε-内酯很少以高选择性形成,而且从来没有没有构象偏差。我们描述了第一个高度对映选择性的 7 - exo -trig iodolactonizations 构象无偏的 ε-不饱和羧酸,由双功能 BAM 催化剂、I 2和 I( III ) 试剂 (PhI(OAc) 2 :PIDA)的不寻常组合影响.



























 京公网安备 11010802027423号
京公网安备 11010802027423号