当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Extensive characterization of choline chloride and its solid–liquid equilibrium with water
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2022-06-08 , DOI: 10.1039/d2cp00377e Ana I M C Lobo Ferreira 1 , Sérgio M Vilas-Boas 2, 3 , Rodrigo M A Silva 1 , Mónia A R Martins 3 , Dinis O Abranches 3 , Paula C R Soares-Santos 3 , Filipe A Almeida Paz 3 , Olga Ferreira 2 , Simão P Pinho 2 , Luís M N B F Santos 1 , João A P Coutinho 3
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2022-06-08 , DOI: 10.1039/d2cp00377e Ana I M C Lobo Ferreira 1 , Sérgio M Vilas-Boas 2, 3 , Rodrigo M A Silva 1 , Mónia A R Martins 3 , Dinis O Abranches 3 , Paula C R Soares-Santos 3 , Filipe A Almeida Paz 3 , Olga Ferreira 2 , Simão P Pinho 2 , Luís M N B F Santos 1 , João A P Coutinho 3
Affiliation
|
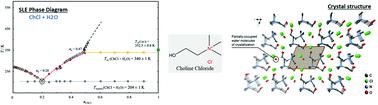
The importance of choline chloride (ChCl) is recognized due to its widespread use in the formulation of deep eutectic solvents. The controlled addition of water in deep eutectic solvents has been proposed to overcome some of the major drawbacks of these solvents, namely their high hygroscopicities and viscosities. Recently, aqueous solutions of ChCl at specific mole ratios have been presented as a novel, low viscous deep eutectic solvent. Nevertheless, these proposals are suggested without any information about the solid–liquid phase diagram of this system or the deviations from the thermodynamic ideality of its precursors. This work contributes significantly to this matter as the phase behavior of pure ChCl and (ChCl + H2O) binary mixtures was investigated by calorimetric and analytical techniques. The thermal behavior and stability of ChCl were studied by polarized light optical microscopy and differential scanning calorimetry, confirming the existence of a solid–solid transition at 352.2 ± 0.6 K. Additionally, heat capacity measurements of pure ChCl (covering both ChCl solid phases) and aqueous solutions of ChCl (xChCl < 0.4) were performed using a heat-flow differential scanning microcalorimeter or a high-precision heat capacity drop calorimeter, allowing the estimation of a heat capacity change of (ChCl) ≈ 39.3 ± 10 J K−1 mol−1, between the hypothetical liquid and the observed crystalline phase at 298.15 K. The solid–liquid phase diagram of the ChCl + water mixture was investigated in the whole concentration range by differential scanning calorimetry and the analytical shake-flask method. The phase diagram obtained for the mixture shows an eutectic temperature of 204 K, at a mole fraction of choline chloride close to xChCl = 0.2, and a shift of the solid–solid transition of ChCl–water mixtures of 10 K below the value observed for pure choline chloride, suggesting the appearance of a new crystalline structure of ChCl in the presence of water, as confirmed by X-ray diffraction. The liquid phase presents significant negative deviations to ideality for water while COSMO-RS predicts a near ideal behaviour for ChCl.
中文翻译:

氯化胆碱的广泛表征及其与水的固液平衡
氯化胆碱 (ChCl) 的重要性因其在深共熔溶剂配方中的广泛应用而得到认可。已经提出在低共熔溶剂中控制添加水以克服这些溶剂的一些主要缺点,即它们的高吸湿性和粘度。最近,特定摩尔比的 ChCl 水溶液作为一种新型的低粘度低共熔溶剂被提出。然而,这些建议是在没有任何关于该系统的固液相图或其前体的热力学理想偏差的信息的情况下提出的。这项工作对这一问题做出了重大贡献,因为纯 ChCl 和 (ChCl + H 2O) 通过量热和分析技术研究二元混合物。通过偏振光学显微镜和差示扫描量热法研究了 ChCl 的热行为和稳定性,证实了在 352.2 ± 0.6 K 处存在固-固转变。此外,纯 ChCl 的热容量测量(包括 ChCl 固相)和使用热流差示扫描微量热仪或高精度热容降量热仪进行ChCl ( x ChCl < 0.4) 的水溶液,允许估计 (ChCl) ≈ 39.3 ± 10 JK -1 mol的热容变化-1,在假设液体和在 298.15 K 观察到的结晶相之间。通过差示扫描量热法和分析摇瓶法研究了整个浓度范围内 ChCl + 水混合物的固液相图。混合物获得的相图显示共晶温度为 204 K,氯化胆碱的摩尔分数接近x ChCl = 0.2,并且 ChCl-水混合物的固-固转变位移低于观察值 10 K对于纯氯化胆碱,表明在水存在下出现了新的 ChCl 晶体结构,如 X 射线衍射所证实。液相对水的理想状态存在显着的负偏差,而 COSMO-RS 预测 ChCl 的行为接近理想。
更新日期:2022-06-08
中文翻译:

氯化胆碱的广泛表征及其与水的固液平衡
氯化胆碱 (ChCl) 的重要性因其在深共熔溶剂配方中的广泛应用而得到认可。已经提出在低共熔溶剂中控制添加水以克服这些溶剂的一些主要缺点,即它们的高吸湿性和粘度。最近,特定摩尔比的 ChCl 水溶液作为一种新型的低粘度低共熔溶剂被提出。然而,这些建议是在没有任何关于该系统的固液相图或其前体的热力学理想偏差的信息的情况下提出的。这项工作对这一问题做出了重大贡献,因为纯 ChCl 和 (ChCl + H 2O) 通过量热和分析技术研究二元混合物。通过偏振光学显微镜和差示扫描量热法研究了 ChCl 的热行为和稳定性,证实了在 352.2 ± 0.6 K 处存在固-固转变。此外,纯 ChCl 的热容量测量(包括 ChCl 固相)和使用热流差示扫描微量热仪或高精度热容降量热仪进行ChCl ( x ChCl < 0.4) 的水溶液,允许估计 (ChCl) ≈ 39.3 ± 10 JK -1 mol的热容变化-1,在假设液体和在 298.15 K 观察到的结晶相之间。通过差示扫描量热法和分析摇瓶法研究了整个浓度范围内 ChCl + 水混合物的固液相图。混合物获得的相图显示共晶温度为 204 K,氯化胆碱的摩尔分数接近x ChCl = 0.2,并且 ChCl-水混合物的固-固转变位移低于观察值 10 K对于纯氯化胆碱,表明在水存在下出现了新的 ChCl 晶体结构,如 X 射线衍射所证实。液相对水的理想状态存在显着的负偏差,而 COSMO-RS 预测 ChCl 的行为接近理想。


























 京公网安备 11010802027423号
京公网安备 11010802027423号