当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nickel-catalysed SET-reduction-based access to functionalized allenes via 1,4-carbohydrogenation of 1,3-enynes with alkyl bromides
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-06-08 , DOI: 10.1039/d2qo00672c Wan Lei 1 , Hong Liu 1 , Yan Li 1 , Yewen Fang 2, 3
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-06-08 , DOI: 10.1039/d2qo00672c Wan Lei 1 , Hong Liu 1 , Yan Li 1 , Yewen Fang 2, 3
Affiliation
|
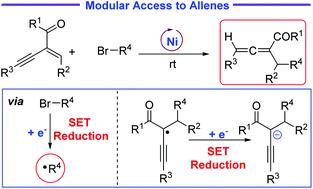
The single-electron-transfer reduction-based radical–polar crossover process has emerged as a useful and powerful strategy for the development of new synthetic transformations. Herein, using SET reduction as the key process, a new nickel-catalysed protocol for the preparation of trisubstituted allenes has been successfully developed via the reactions of enynes with radicals generated from alkyl bromides. According to the results of the deuteration experiment, a mechanism based on the nickel-catalysed reductive radical–polar crossover process has been proposed. The synthetic application of allene has also been demonstrated.
中文翻译:

镍催化 SET 还原通过 1,3-烯炔与烷基溴的 1,4-碳氢化获得功能化丙二烯
基于单电子转移还原的自由基-极性交叉过程已成为开发新合成转化的有用且强大的策略。在此,以 SET 还原为关键过程,通过烯炔与烷基溴产生的自由基的反应,成功开发了一种新的镍催化制备三取代丙二烯的方案。根据氘代实验的结果,提出了一种基于镍催化还原自由基-极性交叉过程的机理。还证明了丙二烯的合成应用。
更新日期:2022-06-08
中文翻译:

镍催化 SET 还原通过 1,3-烯炔与烷基溴的 1,4-碳氢化获得功能化丙二烯
基于单电子转移还原的自由基-极性交叉过程已成为开发新合成转化的有用且强大的策略。在此,以 SET 还原为关键过程,通过烯炔与烷基溴产生的自由基的反应,成功开发了一种新的镍催化制备三取代丙二烯的方案。根据氘代实验的结果,提出了一种基于镍催化还原自由基-极性交叉过程的机理。还证明了丙二烯的合成应用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号