当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
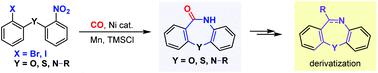
Ni-Catalysed intramolecular reductive aminocarbonylation of 2-haloaryl-tethered nitroarenes for the synthesis of dibenzazepine-based heterocycles
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-06-08 , DOI: 10.1039/d2qo00699e Zhe Feng , Jun-An Ma , Chi Wai Cheung
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-06-08 , DOI: 10.1039/d2qo00699e Zhe Feng , Jun-An Ma , Chi Wai Cheung
|
Herein, we describe an intramolecular reductive aminocarbonylation of haloaryl-tethered nitroarenes with carbon monoxide to access dibenzazepine-based heterocycles. The use of a nickel catalyst and manganese reductant allows the expedient synthesis of structurally diverse dibenzoxazepinones, dibenzothiazepinones, and dibenzodiazepinones, obviating the in-advance transformation of the nitroarene moiety to aniline in a conventional aminocarbonylation process.
中文翻译:

Ni催化2-卤代芳基束缚硝基芳烃分子内还原氨基羰基化合成二苯并氮杂杂环
在此,我们描述了卤代芳基连接的硝基芳烃与一氧化碳的分子内还原氨基羰基化,以获得基于二苯并氮杂的杂环。镍催化剂和锰还原剂的使用允许方便地合成结构多样的二苯并噻嗪酮、二苯并噻嗪酮和二苯并二氮杂卓,避免在常规氨基羰基化过程中预先将硝基芳烃部分转化为苯胺。
更新日期:2022-06-08
中文翻译:

Ni催化2-卤代芳基束缚硝基芳烃分子内还原氨基羰基化合成二苯并氮杂杂环
在此,我们描述了卤代芳基连接的硝基芳烃与一氧化碳的分子内还原氨基羰基化,以获得基于二苯并氮杂的杂环。镍催化剂和锰还原剂的使用允许方便地合成结构多样的二苯并噻嗪酮、二苯并噻嗪酮和二苯并二氮杂卓,避免在常规氨基羰基化过程中预先将硝基芳烃部分转化为苯胺。

























 京公网安备 11010802027423号
京公网安备 11010802027423号