当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Actinium coordination chemistry: A density functional theory study with monodentate and bidentate ligands
Journal of Computational Chemistry ( IF 3 ) Pub Date : 2022-06-06 , DOI: 10.1002/jcc.26929 Josef Tomeček 1, 2 , Cen Li 2 , Georg Schreckenbach 2
Journal of Computational Chemistry ( IF 3 ) Pub Date : 2022-06-06 , DOI: 10.1002/jcc.26929 Josef Tomeček 1, 2 , Cen Li 2 , Georg Schreckenbach 2
Affiliation
|
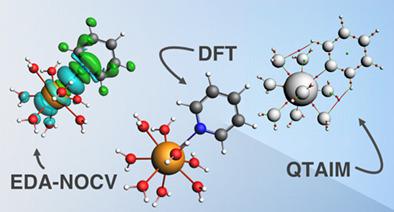
In the current study, the coordination chemistry of nine-coordinate Ac(III) complexes with 35 monodentate and bidentate ligands was investigated using density functional theory (DFT) in terms of their geometries, charges, reaction energies, and bonding interactions. The energy decomposition analysis with naturals orbitals for chemical valence (EDA-NOCV) and the quantum theory of atoms in molecules (QTAIM) were employed as analysis methods. Trivalent Ac exhibits the highest affinities toward hard acids (such as charged oxophilic donors, fluoride), so its classification as a hard acid is justified. Natural population analysis quantified the involvement of 5f orbitals on Ac to be about 30% of total valence electron natural configuration indicating that Ac is a member of the actinide series. Pearson correlation coefficients were used to study the pairwise correlations among the bond lengths, ΔG reaction energies, charges on Ac and donor atoms, and data from EDA-NOCV and QTAIM. Strong correlations and anticorrelations were found between Voronoi charges on donor atoms with ΔG, EDA-NOCV interaction energies and QTAIM bond critical point densities.
中文翻译:

锕配位化学:单齿和双齿配体的密度泛函理论研究
在当前的研究中,使用密度泛函理论 (DFT) 研究了具有 35 个单齿和双齿配体的九配位 Ac(III) 配合物的配位化学,包括其几何形状、电荷、反应能和键合相互作用。采用化学价自然轨道的能量分解分析(EDA-NOCV)和分子中原子的量子理论(QTAIM)作为分析方法。三价 Ac 对硬酸(如带电荷的亲氧供体、氟化物)表现出最高的亲和力,因此将其归类为硬酸是合理的。自然种群分析将 Ac 上 5f 轨道的参与量化为约占总价电子自然构型的 30%,表明 Ac 是锕系元素的成员。G反应能量、Ac 和施主原子的电荷,以及来自 EDA-NOCV 和 QTAIM 的数据。在供体原子上的 Voronoi 电荷与 Δ G、EDA-NOCV 相互作用能和 QTAIM 键临界点密度之间发现了强相关和反相关。
更新日期:2022-06-06
中文翻译:

锕配位化学:单齿和双齿配体的密度泛函理论研究
在当前的研究中,使用密度泛函理论 (DFT) 研究了具有 35 个单齿和双齿配体的九配位 Ac(III) 配合物的配位化学,包括其几何形状、电荷、反应能和键合相互作用。采用化学价自然轨道的能量分解分析(EDA-NOCV)和分子中原子的量子理论(QTAIM)作为分析方法。三价 Ac 对硬酸(如带电荷的亲氧供体、氟化物)表现出最高的亲和力,因此将其归类为硬酸是合理的。自然种群分析将 Ac 上 5f 轨道的参与量化为约占总价电子自然构型的 30%,表明 Ac 是锕系元素的成员。G反应能量、Ac 和施主原子的电荷,以及来自 EDA-NOCV 和 QTAIM 的数据。在供体原子上的 Voronoi 电荷与 Δ G、EDA-NOCV 相互作用能和 QTAIM 键临界点密度之间发现了强相关和反相关。



























 京公网安备 11010802027423号
京公网安备 11010802027423号