当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural analysis of LpqY, a substrate-binding protein from the SugABC transporter of Mycobacterium tuberculosis, provides insights into its trehalose specificity
Acta Crystallographica Section D ( IF 2.2 ) Pub Date : 2022-06-07 , DOI: 10.1107/s2059798322005290 Dipika Sharma 1 , Mandeep Singh 1 , Punit Kaur 1 , Uddipan Das 1
Acta Crystallographica Section D ( IF 2.2 ) Pub Date : 2022-06-07 , DOI: 10.1107/s2059798322005290 Dipika Sharma 1 , Mandeep Singh 1 , Punit Kaur 1 , Uddipan Das 1
Affiliation
|
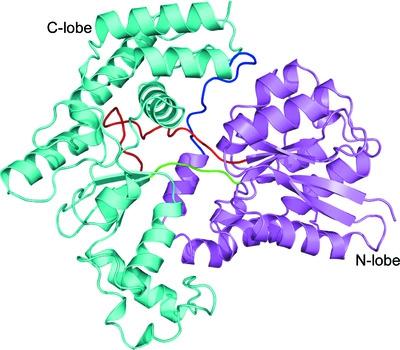
The LpqY-SugABC transporter of Mycobacterium tuberculosis (Mtb) salvages residual trehalose across the cell membrane, which is otherwise lost during the formation of cell-wall glycoconjugates in the periplasm. LpqY, a substrate-binding protein from the SugABC transporter, acts as the primary receptor for the recognition of trehalose, leading to its transport across the cell membrane. Since trehalose is crucial for the survival and virulence of Mtb, trehalose receptors should serve as important targets for novel drug design against tuberculosis. In order to comprehend the detailed architecture and substrate specificity, the first crystal structures of both apo and trehalose-bound forms of M. tuberculosis LpqY (Mtb-LpqY) are presented here at 2.2 and 1.9 Å resolution, respectively. The structure exhibits an N-lobe and C-lobe and is predominantly composed of a globular α/β domain connected by a flexible hinge region concealing a deep binding cleft. Although the trehalose-bound form of Mtb-LpqY revealed an open ligand-bound conformation, the glucose moieties of trehalose are seen to be strongly held in place by direct and water-mediated hydrogen bonds within the binding cavity, producing a Kd of 6.58 ± 1.21 µM. These interactions produce a distinct effect on the stereoselectivity for the α-1,1-glycosidic linkage of trehalose. Consistent with the crystal structure, molecular-dynamics simulations further validated Asp43, Asp97 and Asn151 as key residues responsible for strong and stable interactions throughout a 1 µs time frame, thus capturing trehalose in the binding cavity. Collectively, the results provide detailed insights into how the structure and dynamics of Mtb-LpqY enable it to specifically bind trehalose in a relaxed conformation state.
中文翻译:

LpqY 的结构分析,一种来自结核分枝杆菌 SugABC 转运蛋白的底物结合蛋白,提供了对其海藻糖特异性的见解
结核分枝杆菌(Mtb)的 LpqY-SugABC 转运蛋白可挽救残留在细胞膜上的海藻糖,否则这些海藻糖会在周质中形成细胞壁糖缀合物的过程中丢失。LpqY 是来自 SugABC 转运蛋白的底物结合蛋白,作为识别海藻糖的主要受体,导致其跨细胞膜转运。由于海藻糖对 Mtb 的存活和毒力至关重要,因此海藻糖受体应作为抗结核新药设计的重要靶标。为了理解详细的结构和底物特异性,结核分枝杆菌的载脂蛋白和海藻糖结合形式的第一个晶体结构LpqY (Mtb-LpqY) 分别以 2.2 和 1.9 Å 分辨率呈现。该结构呈现出 N-叶和 C-叶,主要由球状 α/β 结构域组成,该结构域通过隐藏深结合裂隙的柔性铰链区连接。尽管 Mtb-LpqY 的海藻糖结合形式显示出开放的配体结合构象,但海藻糖的葡萄糖部分被结合腔内的直接和水介导的氢键牢固地固定在适当的位置,产生6.58的K d ± 1.21微米. 这些相互作用对海藻糖的 α-1,1-糖苷键的立体选择性产生了明显的影响。与晶体结构一致,分子动力学模拟进一步验证了 Asp43、Asp97 和 Asn151 是负责在 1 µs 时间范围内强而稳定相互作用的关键残基,从而在结合腔中捕获海藻糖。总的来说,这些结果提供了关于 Mtb-LpqY 的结构和动力学如何使其能够在松弛构象状态下特异性结合海藻糖的详细见解。
更新日期:2022-06-07
中文翻译:

LpqY 的结构分析,一种来自结核分枝杆菌 SugABC 转运蛋白的底物结合蛋白,提供了对其海藻糖特异性的见解
结核分枝杆菌(Mtb)的 LpqY-SugABC 转运蛋白可挽救残留在细胞膜上的海藻糖,否则这些海藻糖会在周质中形成细胞壁糖缀合物的过程中丢失。LpqY 是来自 SugABC 转运蛋白的底物结合蛋白,作为识别海藻糖的主要受体,导致其跨细胞膜转运。由于海藻糖对 Mtb 的存活和毒力至关重要,因此海藻糖受体应作为抗结核新药设计的重要靶标。为了理解详细的结构和底物特异性,结核分枝杆菌的载脂蛋白和海藻糖结合形式的第一个晶体结构LpqY (Mtb-LpqY) 分别以 2.2 和 1.9 Å 分辨率呈现。该结构呈现出 N-叶和 C-叶,主要由球状 α/β 结构域组成,该结构域通过隐藏深结合裂隙的柔性铰链区连接。尽管 Mtb-LpqY 的海藻糖结合形式显示出开放的配体结合构象,但海藻糖的葡萄糖部分被结合腔内的直接和水介导的氢键牢固地固定在适当的位置,产生6.58的K d ± 1.21微米. 这些相互作用对海藻糖的 α-1,1-糖苷键的立体选择性产生了明显的影响。与晶体结构一致,分子动力学模拟进一步验证了 Asp43、Asp97 和 Asn151 是负责在 1 µs 时间范围内强而稳定相互作用的关键残基,从而在结合腔中捕获海藻糖。总的来说,这些结果提供了关于 Mtb-LpqY 的结构和动力学如何使其能够在松弛构象状态下特异性结合海藻糖的详细见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号