Acta Pharmaceutica Sinica B ( IF 14.5 ) Pub Date : 2022-06-03 , DOI: 10.1016/j.apsb.2022.05.031 Meng Chen 1, 2 , Zhengyan Guo 1, 3 , Jinyuan Sun 1, 2 , Wei Tang 1, 2 , Min Wang 1, 4 , Yue Tang 1 , Pengwei Li 1 , Bian Wu 1, 2 , Yihua Chen 1, 2
|
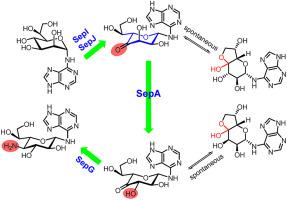
l-Heptopyranoses are important components of bacterial polysaccharides and biological active secondary metabolites like septacidin (SEP), which represents a group of nucleoside antibiotics with antitumor, antifungal, and pain-relief activities. However, little is known about the formation mechanisms of those l-heptose moieties. In this study, we deciphered the biosynthetic pathway of the l,l-gluco-heptosamine moiety in SEPs by functional characterizing four genes and proposed that SepI initiates the process by oxidizing the 4′-hydroxyl of l-glycero-α-d-manno-heptose moiety of SEP-328 (2) to a keto group. Subsequently, SepJ (C5 epimerase) and SepA (C3 epimerase) shape the 4′-keto-l-heptopyranose moiety by sequential epimerization reactions. At the last step, an aminotransferase SepG installs the 4′-amino group of the l,l-gluco-heptosamine moiety to generate SEP-327 (3). An interesting phenomenon is that the SEP intermediates with 4′-keto-l-heptopyranose moieties exist as special bicyclic sugars with hemiacetal-hemiketal structures. Notably, l-pyranose is usually converted from d-pyranose by bifunctional C3/C5 epimerase. SepA is an unprecedented monofunctional l-pyranose C3 epimerase. Further in silico and experimental studies revealed that it represents an overlooked metal dependent-sugar epimerase family bearing vicinal oxygen chelate (VOC) architecture.
中文翻译:

深入了解 septacidin l-heptosamine 部分的生物合成揭示了一种 VOC 家族糖差向异构酶
l-吡喃庚糖是细菌多糖和生物活性次级代谢产物的重要组成部分,如 septacidin (SEP),代表一组具有抗肿瘤、抗真菌和镇痛活性的核苷类抗生素。然而,人们对这些l-庚糖部分的形成机制知之甚少。在这项研究中,我们通过对四个基因进行功能表征,破译了 SEP 中l,l-葡糖胺部分的生物合成途径,并提出 SepI 通过氧化l-甘油-α - d-甘露糖的 4'-羟基来启动该过程-SEP-328 的庚糖部分 ( 2) 到酮组。随后,SepJ(C5 差向异构酶)和 SepA(C3 差向异构酶)通过顺序差向异构化反应形成 4'-酮基-1-吡喃庚糖部分。在最后一步,氨基转移酶 SepG 安装l,l-葡糖-庚糖胺部分的 4'-氨基以生成 SEP-327 ( 3 )。一个有趣的现象是,具有 4'-酮基-l-吡喃庚糖部分的 SEP 中间体以具有半缩醛-半缩醛结构的特殊双环糖形式存在。值得注意的是,l-吡喃糖通常通过双功能 C3/C5 差向异构酶从d-吡喃糖转化而来。SepA 是一种前所未有的单功能l-吡喃糖 C3 差向异构酶。进一步在计算机中和实验研究表明,它代表了一个被忽视的金属依赖性糖差向异构酶家族,具有邻位氧螯合物 (VOC) 结构。


























 京公网安备 11010802027423号
京公网安备 11010802027423号