Journal of Catalysis ( IF 7.3 ) Pub Date : 2022-06-04 , DOI: 10.1016/j.jcat.2022.06.002 Chunzheng Wang , Bin Liu , Panyue Liu , Ke Huang , Ningkun Xu , Hailing Guo , Peng Bai , Lixia Ling , Xinmei Liu , Svetlana Mintova
|
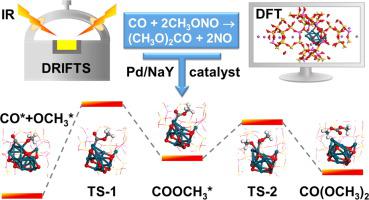
Gas-phase indirect oxidative carbonylation of methanol to dimethyl carbonate (DMC) has been industrialized, but the reaction mechanism is still ambiguous. In this work, the reaction mechanism of DMC synthesis using a NaY zeolite catalyst doped with 1.0 wt% Pd was revealed by combining in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) results with density functional theory (DFT) calculations. Two key reaction intermediates CO* (*, a surface site) and COOCH3* were identified through the adsorption of a probe molecule of methyl chloroformate (CH3OCOCl), and characterized by steady-state, dynamic-pulse and time-resolved transient DRIFTS experiments. The CO* intermediate is predominant on the catalyst surface when the reaction reached steady-state. The DFT results also showed that the inclusion of OCH3* into CO* had the highest energy barrier of 150.1 kJ mol−1. This verified that the formation of COOCH3* is the rate-determining step for the DMC synthesis. A Langmuir-Hinshelwood mechanism including the fast formation of CO*, and rate-determining insertion of OCH3* into CO* toward generation of COOCH3* to yield DMC was proposed.
中文翻译:

阐明甲醇在 Pd/NaY 催化剂上间接氧化羰基化制碳酸二甲酯的反应机理:反应中间体的直接鉴定
甲醇气相间接氧化羰基化制碳酸二甲酯(DMC)已实现工业化,但反应机理尚不明确。在这项工作中,通过将原位漫反射红外傅里叶变换光谱 (DRIFTS) 结果与密度泛函理论 (DFT) 计算相结合,揭示了使用掺杂 1.0 wt% Pd 的 NaY 沸石催化剂合成 DMC 的反应机理。通过吸附氯甲酸甲酯(CH 3OCOCl),并以稳态、动态脉冲和时间分辨瞬态漂移实验为特征。当反应达到稳态时,CO* 中间体在催化剂表面占优势。DFT结果还表明,将OCH 3 * 包含在CO* 中具有最高的能垒,为150.1 kJ mol -1。这证实了COOCH 3 * 的形成是DMC合成的速率决定步骤。提出了一种 Langmuir-Hinshelwood 机制,包括 CO* 的快速形成,以及 OCH 3 * 到 CO* 中的速率决定性插入,以产生 COOCH 3 * 以产生 DMC。


























 京公网安备 11010802027423号
京公网安备 11010802027423号