当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Selectivity through Targeted Protein Degradation (TPD)
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2022-06-03 , DOI: 10.1021/acs.jmedchem.2c00397 Ariamala Gopalsamy 1
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2022-06-03 , DOI: 10.1021/acs.jmedchem.2c00397 Ariamala Gopalsamy 1
Affiliation
|
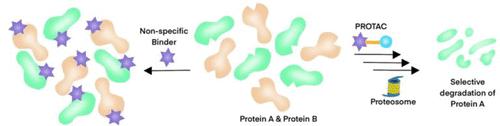
Targeted protein degradation has become a reliable tool in the medicinal chemist’s toolbox, as seen with rapid progression of PROTACs (proteolysis targeting chimeras) to clinic. Degraders have unique advantages to target proteins with no functional consequence or scaffolding function to achieve the desired phenotype. In some cases, selectivity was achieved among closely related targets. While the prospective design of degraders to achieve selectivity remains empirical, this Miniperspective analyzes some reported examples to gather key factors that are hypothesized to contribute to selectivity. Ternary complex conformation to access key lysine residues stands out as a potential key contributor. However, protein and E3 ligase expression levels, differential tissue expression, resynthesis rate, ubiquitination rate, and the stability of the ternary complex formed all have the potential to play a significant role. With continued progress in ternary structure determination along with several predictive modeling methods, a rational approach to achieve degradation and selectivity is tantalizingly close.
中文翻译:

通过靶向蛋白质降解 (TPD) 进行选择性
靶向蛋白质降解已成为药物化学家工具箱中的可靠工具,正如 PROTAC(蛋白水解靶向嵌合体)快速发展到临床所见。降解剂对靶向蛋白质具有独特的优势,没有功能后果或支架功能来实现所需的表型。在某些情况下,在密切相关的目标中实现了选择性。虽然实现选择性的降解剂的前瞻性设计仍然是经验性的,但本 Miniperspective 分析了一些报告的示例,以收集假设有助于选择性的关键因素。获得关键赖氨酸残基的三元复合物构象是潜在的关键贡献者。然而,蛋白质和 E3 连接酶表达水平、差异组织表达、再合成率、泛素化率、而所形成的三元配合物的稳定性都有可能发挥重大作用。随着三元结构测定以及几种预测建模方法的不断进步,实现降解和选择性的合理方法非常接近。
更新日期:2022-06-03
中文翻译:

通过靶向蛋白质降解 (TPD) 进行选择性
靶向蛋白质降解已成为药物化学家工具箱中的可靠工具,正如 PROTAC(蛋白水解靶向嵌合体)快速发展到临床所见。降解剂对靶向蛋白质具有独特的优势,没有功能后果或支架功能来实现所需的表型。在某些情况下,在密切相关的目标中实现了选择性。虽然实现选择性的降解剂的前瞻性设计仍然是经验性的,但本 Miniperspective 分析了一些报告的示例,以收集假设有助于选择性的关键因素。获得关键赖氨酸残基的三元复合物构象是潜在的关键贡献者。然而,蛋白质和 E3 连接酶表达水平、差异组织表达、再合成率、泛素化率、而所形成的三元配合物的稳定性都有可能发挥重大作用。随着三元结构测定以及几种预测建模方法的不断进步,实现降解和选择性的合理方法非常接近。



























 京公网安备 11010802027423号
京公网安备 11010802027423号