Structure ( IF 5.7 ) Pub Date : 2022-06-03 , DOI: 10.1016/j.str.2022.05.010 Giulia Morra 1 , Asghar M Razavi 2 , Anant K Menon 3 , George Khelashvili 4
|
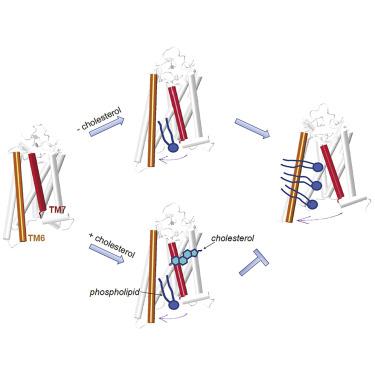
Class A (rhodopsin-like) G protein-coupled receptors (GPCRs) are constitutive phospholipid scramblases as evinced after their reconstitution into liposomes. Yet phospholipid scrambling is not detectable in the resting plasma membrane of mammalian cells that is replete with GPCRs. We considered whether cholesterol, a prominent component of the plasma membrane, limits the ability of GPCRs to scramble lipids. Our previous Markov State Model (MSM) analysis of molecular dynamics simulations of membrane-embedded opsin indicated that phospholipid headgroups traverse a dynamically revealed hydrophilic groove between transmembrane helices (TM) 6 and 7 while their tails remain in the bilayer. Here, we present comparative MSM analyses of 150-μs simulations of opsin in cholesterol-free and cholesterol-rich membranes. Our analyses reveal that cholesterol inhibits phospholipid scrambling by occupying the TM6/7 interface and stabilizing the closed groove conformation while itself undergoing flip-flop. This mechanism may explain the inability of GPCRs to scramble lipids at the plasma membrane.
中文翻译:

胆固醇占据脂质易位途径,通过 G 蛋白偶联受体阻断磷脂加扰
A 类(视紫红质样)G 蛋白偶联受体 (GPCR) 是组成型磷脂混杂酶,如在脂质体中重建后所示。然而,在充满 GPCR 的哺乳动物细胞的静息质膜中检测不到磷脂扰乱。我们考虑了质膜的重要组成部分胆固醇是否会限制 GPCR 扰乱脂质的能力。我们之前对膜嵌入视蛋白的分子动力学模拟进行的马尔可夫状态模型 (MSM) 分析表明,磷脂头基穿过跨膜螺旋 (TM) 6 和 7 之间动态显示的亲水凹槽,而其尾部保留在双层中。在这里,我们对无胆固醇和富含胆固醇的膜中视蛋白的 150 μs 模拟进行了比较 MSM 分析。我们的分析表明,胆固醇通过占据 TM6/7 界面并稳定封闭凹槽构象来抑制磷脂加扰,同时自身发生翻转。这种机制可以解释 GPCR 无法扰乱质膜上的脂质的原因。


























 京公网安备 11010802027423号
京公网安备 11010802027423号