Phytomedicine ( IF 7.9 ) Pub Date : 2022-06-03 , DOI: 10.1016/j.phymed.2022.154235 Wenling Zhou 1 , Xu Yan 1 , Yuanyuan Zhai 1 , Hao Liu 2 , Lingling Guan 2 , Yuan Qiao 2 , Jizhi Jiang 3 , Liang Peng 2
|
Background
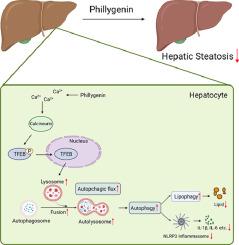
: Lipophagy is an autophagic process, which delivers the intracellular lipid droplets to the lysosomes for degradation. Recent studies revealed that the impairment of lysosomal biogenesis and autophagic flux led to dysregulation of lipophagy in hepatocytes, which exacerbated the development of nonalcoholic fatty liver disease (NAFLD). Therefore, agents restoring autophagic flux and lipophagy in hepatocytes may have therapeutic potential against this increasingly prevalent disease. Phillygenin (PHI), a lignin extracted from Forsythia suspense, exerts hepatoprotective and anti-inflammatory effects. However, the effect of PHI on NAFLD remains unknown.
Purpose
: This study aimed to investigate the protective effect of PHI on NAFLD and elucidate the underlying mechanism.
Methods
: The effects of PHI were examined in palmitate (PA)-stimulated AML12 cells and primary hepatocytes, as well as in NAFLD mice induced by a high-fat diet (HFD). We also used transcription factor EB (TFEB) knockdown hepatocytes and hepatocyte-specific TFEB knockout (TFEBΔhep) mice for mechanistic studies. In vivo and in vitro studies were performed using western blots, immunofluorescence techniques, and transmission electron microscopy.
Results
: Our results indicated that autophagic flux and lysosome biogenesis in PA-stimulated hepatocytes were impaired. PHI alleviated lipid deposition by increasing lysosomal biogenesis and autophagic flux. It also stimulated the release of endoplasmic reticulum Ca2+ to activate calcineurin, which regulated TFEB dephosphorylation and nuclear translocation, and promoted lysosomal biogenesis. In addition, PHI blocked the NLRP3 inflammasome pathway and improved hepatocyte inflammation in an autophagy-dependent manner. Consistent with the in vitro results, PHI improved hepatic steatosis and inflammation in HFD mice, but these beneficial effects were eliminated in hepatocyte-specific TFEB knockout mice.
Conclusion
: Despite PHI has been reported to have anti-hepatic fibrosis effects, whether it has a hepatoprotective effects against NAFLD and the underlying molecular mechanism remain unclear. Herein, we found that PHI restored lipophagy and suppressed lipid accumulation and inflammation by regulating the Ca2+-calcineurin-TFEB axis in hepatocytes. Thus, PHI represents a therapeutic candidate for the treatment of NAFLD.
中文翻译:

Phillygenin 通过 TFEB 介导的溶酶体生物合成和脂肪吞噬改善非酒精性脂肪肝
背景
:自噬是一种自噬过程,它将细胞内的脂滴输送到溶酶体进行降解。最近的研究表明,溶酶体生物合成和自噬通量的损害导致肝细胞中脂肪吞噬的失调,从而加剧了非酒精性脂肪肝病 (NAFLD) 的发展。因此,在肝细胞中恢复自噬通量和脂肪吞噬的药物可能对这种日益流行的疾病具有治疗潜力。Phillygenin (PHI) 是一种从连翘中提取的木质素,具有保肝和抗炎作用。然而,PHI 对 NAFLD 的影响仍然未知。
目的
: 本研究旨在探讨 PHI 对 NAFLD 的保护作用并阐明其潜在机制。
方法
:在棕榈酸酯 (PA) 刺激的 AML12 细胞和原代肝细胞以及高脂饮食 (HFD) 诱导的 NAFLD 小鼠中检测了 PHI 的影响。我们还使用转录因子 EB (TFEB) 敲低肝细胞和肝细胞特异性 TFEB 敲除 (TFEB Δhep ) 小鼠进行机制研究。使用蛋白质印迹、免疫荧光技术和透射电子显微镜进行体内和体外研究。
结果
:我们的结果表明,PA 刺激的肝细胞中的自噬通量和溶酶体生物发生受损。PHI 通过增加溶酶体生物合成和自噬通量来减轻脂质沉积。它还刺激内质网Ca 2+释放,激活钙调神经磷酸酶,调节TFEB去磷酸化和核转位,促进溶酶体生物合成。此外,PHI 阻断 NLRP3 炎性体通路,并以自噬依赖性方式改善肝细胞炎症。与体外结果一致,PHI 改善了 HFD 小鼠的肝脏脂肪变性和炎症,但这些有益效果在肝细胞特异性 TFEB 敲除小鼠中被消除。
结论
: 尽管已报道 PHI 具有抗肝纤维化作用,但它是否具有抗 NAFLD 的肝保护作用以及潜在的分子机制仍不清楚。在此,我们发现 PHI 通过调节肝细胞中的 Ca 2+ -calcineurin-TFEB 轴来恢复脂肪吞噬并抑制脂质积累和炎症。因此,PHI 代表了治疗 NAFLD 的治疗候选者。



























 京公网安备 11010802027423号
京公网安备 11010802027423号