当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Insights on JAK2 Modulation by Potent, Selective, and Cell-Permeable Pseudokinase-Domain Ligands
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2022-06-02 , DOI: 10.1021/acs.jmedchem.2c00283 Maria-Elena Liosi 1 , Joseph A Ippolito 1 , Sean P Henry 1 , Stefan G Krimmer 1, 2 , Ana S Newton 1 , Kara J Cutrona 1 , Rene A Olivarez 1 , Jyotidarsini Mohanty 2 , Joseph Schlessinger 2 , William L Jorgensen 1
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2022-06-02 , DOI: 10.1021/acs.jmedchem.2c00283 Maria-Elena Liosi 1 , Joseph A Ippolito 1 , Sean P Henry 1 , Stefan G Krimmer 1, 2 , Ana S Newton 1 , Kara J Cutrona 1 , Rene A Olivarez 1 , Jyotidarsini Mohanty 2 , Joseph Schlessinger 2 , William L Jorgensen 1
Affiliation
|
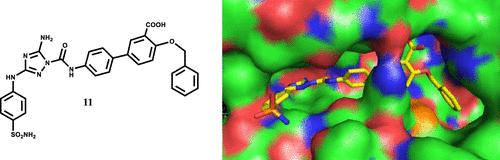
JAK2 is a non-receptor tyrosine kinase that regulates hematopoiesis through the JAK-STAT pathway. The pseudokinase domain (JH2) is an important regulator of the activity of the kinase domain (JH1). V617F mutation in JH2 has been associated with the pathogenesis of various myeloproliferative neoplasms, but JAK2 JH2 has been poorly explored as a pharmacological target. In light of this, we aimed to develop JAK2 JH2 binders that could selectively target JH2 over JH1 and test their capacity to modulate JAK2 activity in cells. Toward this goal, we optimized a diaminotriazole lead compound into potent, selective, and cell-permeable JH2 binders leveraging computational design, synthesis, binding affinity measurements for the JH1, JH2 WT, and JH2 V617F domains, permeability measurements, crystallography, and cell assays. Optimized diaminotriazoles are capable of inhibiting STAT5 phosphorylation in both WT and V617F JAK2 in cells.
中文翻译:

强效、选择性和细胞渗透性假激酶域配体对 JAK2 调制的见解
JAK2 是一种非受体酪氨酸激酶,通过 JAK-STAT 通路调节造血功能。假激酶结构域 (JH2) 是激酶结构域 (JH1) 活性的重要调节因子。JH2 中的 V617F 突变与各种骨髓增生性肿瘤的发病机制有关,但 JAK2 JH2 作为药理学靶点的探索很少。鉴于此,我们的目标是开发 JAK2 JH2 结合物,可以选择性地靶向 JH2 而不是 JH1,并测试它们调节细胞中 JAK2 活性的能力。为实现这一目标,我们利用 JH1、JH2 WT 和 JH2 V617F 结构域的计算设计、合成、结合亲和力测量、渗透性测量、晶体学和细胞分析,将二氨基三唑先导化合物优化为有效、选择性和细胞渗透性 JH2 结合剂.
更新日期:2022-06-02
中文翻译:

强效、选择性和细胞渗透性假激酶域配体对 JAK2 调制的见解
JAK2 是一种非受体酪氨酸激酶,通过 JAK-STAT 通路调节造血功能。假激酶结构域 (JH2) 是激酶结构域 (JH1) 活性的重要调节因子。JH2 中的 V617F 突变与各种骨髓增生性肿瘤的发病机制有关,但 JAK2 JH2 作为药理学靶点的探索很少。鉴于此,我们的目标是开发 JAK2 JH2 结合物,可以选择性地靶向 JH2 而不是 JH1,并测试它们调节细胞中 JAK2 活性的能力。为实现这一目标,我们利用 JH1、JH2 WT 和 JH2 V617F 结构域的计算设计、合成、结合亲和力测量、渗透性测量、晶体学和细胞分析,将二氨基三唑先导化合物优化为有效、选择性和细胞渗透性 JH2 结合剂.


























 京公网安备 11010802027423号
京公网安备 11010802027423号