当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hydration of divalent lanthanides, Sm2+ and Eu2+: A molecular dynamics study with polarizable AMOEBA force field
Journal of Computational Chemistry ( IF 3 ) Pub Date : 2022-06-01 , DOI: 10.1002/jcc.26933 Hesam Arabzadeh 1 , Chengwen Liu 2 , Orlando Acevedo 3 , Pengyu Ren 2 , Wei Yang 1, 4 , Thomas Albrecht-Schönzart 1
Journal of Computational Chemistry ( IF 3 ) Pub Date : 2022-06-01 , DOI: 10.1002/jcc.26933 Hesam Arabzadeh 1 , Chengwen Liu 2 , Orlando Acevedo 3 , Pengyu Ren 2 , Wei Yang 1, 4 , Thomas Albrecht-Schönzart 1
Affiliation
|
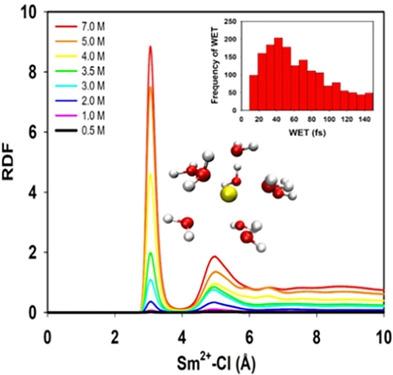
The chemistry of divalent lanthanides, Ln2+, is a growing sub-field of heavy element chemistry owing to new synthetic approaches. However, some theoretical aspects of these unusual cations are currently underdeveloped, especially as they relate to their dynamic properties in solution. In this work, we address the hydration of two of the classical Ln2+ cations, Sm2+ and Eu2+, using atomic multipole optimized energetic for biomolecular applications (AMOEBA) force fields. These cations have not been parameterized to date with AMOEBA, and few studies are available because of their instability with respect to oxidation in aqueous media. Coordination numbers (CN's) of 8.2 and 8.1 respectively for Sm2+ and Eu2+, and 8.8 for both Sm3+ and Eu3+ have been obtained and are in good agreement with the few available AIMD and X-ray absorption fine structures studies. The decreased CN of Ln2+ compared with Ln3+ arises from progressive water exchange events that indicates the gradual stabilization of 8-coordinate structures with respect to 9-coordinate geometries. Moreover, the effects of the chloride counter anions on the coordination of Ln2+ cations have been studied at different chloride concentrations in this work. Lastly, water exchange times of Ln2+ cations have been calculated to provide a comprehensive understanding of the behavior of Eu2+ and Sm2+ in aqueous chloride media.
中文翻译:

二价镧系元素、Sm2+ 和 Eu2+ 的水合:可极化 AMOEBA 力场的分子动力学研究
由于出现了新的合成方法,二价镧系元素 Ln 2+的化学成为重元素化学的一个不断发展的子领域。然而,这些不寻常阳离子的一些理论方面目前尚不完善,尤其是当它们与它们在溶液中的动态特性有关时。在这项工作中,我们使用原子多极优化能量生物分子应用 (AMOEBA) 力场解决了两种经典 Ln 2+阳离子 Sm 2+和 Eu 2+的水合作用。迄今为止,这些阳离子尚未用 AMOEBA 进行参数化,并且由于它们在水性介质中的氧化不稳定,因此很少有研究可用。Sm 2+和 Eu的配位数 (CN) 分别为 8.2 和 8.1已获得Sm 3+和 Eu 3+的2+和 8.8 ,并且与少数可用的 AIMD 和 X 射线吸收精细结构研究非常一致。与Ln 3+相比,Ln 2+的 CN 降低是由渐进的水交换事件引起的,这表明 8 坐标结构相对于 9 坐标几何形状逐渐稳定。此外,本研究还研究了不同氯化物浓度下氯化物抗衡阴离子对 Ln 2+阳离子配位的影响。最后,计算了Ln 2+阳离子的水交换时间,以全面了解 Eu 2+和 Sm的行为2+在含水氯化物介质中。
更新日期:2022-06-01
中文翻译:

二价镧系元素、Sm2+ 和 Eu2+ 的水合:可极化 AMOEBA 力场的分子动力学研究
由于出现了新的合成方法,二价镧系元素 Ln 2+的化学成为重元素化学的一个不断发展的子领域。然而,这些不寻常阳离子的一些理论方面目前尚不完善,尤其是当它们与它们在溶液中的动态特性有关时。在这项工作中,我们使用原子多极优化能量生物分子应用 (AMOEBA) 力场解决了两种经典 Ln 2+阳离子 Sm 2+和 Eu 2+的水合作用。迄今为止,这些阳离子尚未用 AMOEBA 进行参数化,并且由于它们在水性介质中的氧化不稳定,因此很少有研究可用。Sm 2+和 Eu的配位数 (CN) 分别为 8.2 和 8.1已获得Sm 3+和 Eu 3+的2+和 8.8 ,并且与少数可用的 AIMD 和 X 射线吸收精细结构研究非常一致。与Ln 3+相比,Ln 2+的 CN 降低是由渐进的水交换事件引起的,这表明 8 坐标结构相对于 9 坐标几何形状逐渐稳定。此外,本研究还研究了不同氯化物浓度下氯化物抗衡阴离子对 Ln 2+阳离子配位的影响。最后,计算了Ln 2+阳离子的水交换时间,以全面了解 Eu 2+和 Sm的行为2+在含水氯化物介质中。


























 京公网安备 11010802027423号
京公网安备 11010802027423号