Structure ( IF 5.7 ) Pub Date : 2022-05-30 , DOI: 10.1016/j.str.2022.05.003 Yi Zhang 1 , Christina G Towers 2 , Upendra K Singh 3 , Jiuyang Liu 4 , Maria Håkansson 5 , Derek T Logan 5 , Oreola Donini 6 , Tatiana G Kutateladze 4
|
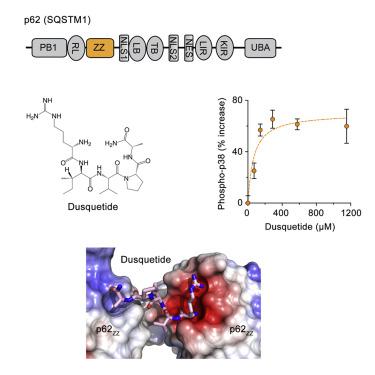
SQSTM1/p62 is an autophagic receptor that plays a major role in mediating stress and innate immune responses. Preclinical studies identified p62 as a target of the prototype innate defense regulator (IDR); however, the molecular mechanism of this process remains unclear. Here, we describe the structural basis and biological consequences of the interaction of p62 with the next generation of IDRs, dusquetide. Both electrostatic and hydrophobic contacts drive the formation of the complex between dusquetide and the ZZ domain of p62. We show that dusquetide penetrates the cell membrane and associates with p62 in vivo. Dusquetide binding modulates the p62-RIP1 complex, increases p38 phosphorylation, and enhances CEBP/B expression without activating autophagy. Our findings provide molecular details underlying the IDR action that may help in the development of new strategies to pharmacologically target p62.
中文翻译:

Dusquetide 通过与 p62 结合调节先天免疫反应
SQSTM1/p62 是一种自噬受体,在介导应激和先天免疫反应中发挥着重要作用。临床前研究将 p62 确定为原型先天防御调节因子 (IDR) 的靶标;然而,这一过程的分子机制仍不清楚。在这里,我们描述了 p62 与下一代 IDRs dusquetide 相互作用的结构基础和生物学后果。静电和疏水接触都驱动 dusquetide 和 p62 ZZ 结构域之间复合物的形成。我们发现 dusquetide 可穿透细胞膜并在体内与 p62 结合。Dusquetide 结合调节 p62-RIP1 复合物,增加 p38 磷酸化,并增强 CEBP/B 表达,而不激活自噬。我们的研究结果提供了 IDR 作用的分子细节,可能有助于开发药理学靶向 p62 的新策略。


























 京公网安备 11010802027423号
京公网安备 11010802027423号