当前位置:
X-MOL 学术
›
J. Chem. Theory Comput.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hydration of Linear Alkanes is Governed by the Small Length-Scale Hydrophobic Effect
Journal of Chemical Theory and Computation ( IF 5.5 ) Pub Date : 2022-06-01 , DOI: 10.1021/acs.jctc.2c00219 Himanshu Singh 1 , Sumit Sharma 1
Journal of Chemical Theory and Computation ( IF 5.5 ) Pub Date : 2022-06-01 , DOI: 10.1021/acs.jctc.2c00219 Himanshu Singh 1 , Sumit Sharma 1
Affiliation
|
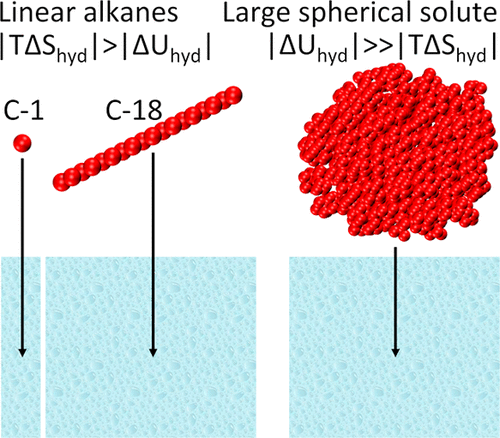
Length-scale dependence of the hydrophobic effect is well understood for apolar spherical solutes: for small solutes (diameter, d ≲ 0.8 nm), the hydration free energy is entropically driven, while for larger solutes (d ≳ 2 nm), it is enthalpically driven. The nature of the hydrophobic effect in the case of anisotropic molecules such as linear alkanes is not understood yet. In this work, we have calculated the hydration free energy of linear alkanes going from methane to octadecane and of a spherical decane droplet of d ≈ 3 nm using molecular simulations. We show that the hydration free energies of alkanes, irrespective of their size, are governed by the small length-scale hydrophobic effect. That is, unlike the case of large spherical solutes, the hydration free energies of linear alkanes are entropically driven.
中文翻译:

直链烷烃的水合受小长度尺度疏水效应控制
对于非极性球形溶质,疏水效应的长度尺度依赖性很容易理解:对于小溶质(直径,d ≲ 0.8 nm),水合自由能是熵驱动的,而对于较大的溶质(d ≳ 2 nm),它是焓驱动的驱动。在各向异性分子(如直链烷烃)的情况下,疏水效应的性质尚不清楚。在这项工作中,我们计算了从甲烷到十八烷的直链烷烃和d的球形癸烷液滴的水合自由能≈ 3 nm 使用分子模拟。我们表明烷烃的水合自由能,无论其大小如何,都受小长度尺度疏水效应的控制。也就是说,与大球形溶质的情况不同,直链烷烃的水合自由能是熵驱动的。
更新日期:2022-06-01
中文翻译:

直链烷烃的水合受小长度尺度疏水效应控制
对于非极性球形溶质,疏水效应的长度尺度依赖性很容易理解:对于小溶质(直径,d ≲ 0.8 nm),水合自由能是熵驱动的,而对于较大的溶质(d ≳ 2 nm),它是焓驱动的驱动。在各向异性分子(如直链烷烃)的情况下,疏水效应的性质尚不清楚。在这项工作中,我们计算了从甲烷到十八烷的直链烷烃和d的球形癸烷液滴的水合自由能≈ 3 nm 使用分子模拟。我们表明烷烃的水合自由能,无论其大小如何,都受小长度尺度疏水效应的控制。也就是说,与大球形溶质的情况不同,直链烷烃的水合自由能是熵驱动的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号