当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An N-heterocyclic carbene-catalyzed enantioselective [3 + 2] annulation of enals with propargylic imines: access to γ,γ-disubstituted pyrrolidin-2-ones bearing quaternary stereogenic centers
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-05-31 , DOI: 10.1039/d2qo00350c Jianming Zhang, Zheng Liang, Simiao Zhang, Lei Chen, Xiaoxue Wang, Yuchan Wang, Jie Feng, Tao Lu, Ding Du, Jian Gao
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-05-31 , DOI: 10.1039/d2qo00350c Jianming Zhang, Zheng Liang, Simiao Zhang, Lei Chen, Xiaoxue Wang, Yuchan Wang, Jie Feng, Tao Lu, Ding Du, Jian Gao
|
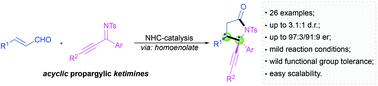
An N-heterocyclic carbene-catalyzed asymmetric [3 + 2] annulation of enals with propargylic ketimines for the facile and enantioselective construction of γ,γ-disubstituted pyrrolidin-2-ones has been developed. This potocol was featured with mild reaction conditions, wild functional group tolerance, easy scalability, and high level of enantioselectivity, which also enriched the chemistry of NHC-bound homoenolates involved synthesis of γ-lactams bearing γ-quaternary stereogenic centers from acyclic kitimimes.
中文翻译:

N-杂环卡宾催化的对映选择性[3 + 2]烯醛与炔丙基亚胺的环化:获得带有季立体中心的γ,γ-二取代吡咯烷-2-酮
已经开发了一种N-杂环卡宾催化的不对称 [3 + 2] 烯醛与炔丙基酮亚胺的环化,用于轻松和对映选择性地构建 γ,γ-二取代吡咯烷-2-酮。该方案具有反应条件温和、官能团耐受性强、易于扩展和对映选择性高的特点,这也丰富了 NHC 结合的均烯醇化物的化学性质,包括从无环基亚甲基合成带有 γ-季铵立体中心的 γ-内酰胺。
更新日期:2022-05-31
中文翻译:

N-杂环卡宾催化的对映选择性[3 + 2]烯醛与炔丙基亚胺的环化:获得带有季立体中心的γ,γ-二取代吡咯烷-2-酮
已经开发了一种N-杂环卡宾催化的不对称 [3 + 2] 烯醛与炔丙基酮亚胺的环化,用于轻松和对映选择性地构建 γ,γ-二取代吡咯烷-2-酮。该方案具有反应条件温和、官能团耐受性强、易于扩展和对映选择性高的特点,这也丰富了 NHC 结合的均烯醇化物的化学性质,包括从无环基亚甲基合成带有 γ-季铵立体中心的 γ-内酰胺。


























 京公网安备 11010802027423号
京公网安备 11010802027423号