当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
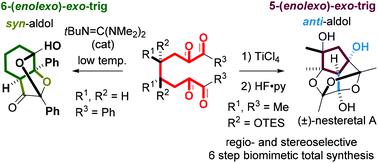
Cascade cyclization of 1,2,7,8-tetraones and total synthesis of (±)-nesteretal A
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-05-31 , DOI: 10.1039/d2qo00740a Tomoka Dentani , Ayano Kawachi , Misaki Kato , Tomoyuki Yoshimura , Jun-ichi Matsuo
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-05-31 , DOI: 10.1039/d2qo00740a Tomoka Dentani , Ayano Kawachi , Misaki Kato , Tomoyuki Yoshimura , Jun-ichi Matsuo
|
For the short synthesis of highly oxidized complex molecules, the regioselectivity and stereoselectivity of intramolecular domino aldol cyclization/acetalizations of 1,2,7,8-tetraones were investigated by using catalytic amounts of Brønsted bases, Brønsted acids, and Lewis acids. Good enantioselectivities (up to 76% ee) were observed in direct catalytic asymmetric cyclization of a 1,2,7,8-tetraone with chiral organocatalysts. The total synthesis of (±)-nesteretal A, a highly oxidized cage molecule, was accomplished in 7 steps by using TiCl4-promoted anti-selective aldol cyclization of a symmetrical 1,2,7,8-tetraone as a key reaction.
中文翻译:

1,2,7,8-四酮的级联环化和 (±)-nesteretal A 的全合成
对于高度氧化的复杂分子的短合成,通过使用催化量的布朗斯台德碱、布朗斯台德酸和路易斯酸,研究了 1,2,7,8-四酮的分子内多米诺醛醇环化/缩醛化的区域选择性和立体选择性。在 1,2,7,8-四酮与手性有机催化剂的直接催化不对称环化中观察到良好的对映选择性(高达 76% ee)。(±)-nesteretal A(一种高度氧化的笼形分子)的全合成通过使用 TiCl 4促进的对称1,2,7,8-四酮的抗选择性羟醛环化作为关键反应分 7 步完成。
更新日期:2022-05-31
中文翻译:

1,2,7,8-四酮的级联环化和 (±)-nesteretal A 的全合成
对于高度氧化的复杂分子的短合成,通过使用催化量的布朗斯台德碱、布朗斯台德酸和路易斯酸,研究了 1,2,7,8-四酮的分子内多米诺醛醇环化/缩醛化的区域选择性和立体选择性。在 1,2,7,8-四酮与手性有机催化剂的直接催化不对称环化中观察到良好的对映选择性(高达 76% ee)。(±)-nesteretal A(一种高度氧化的笼形分子)的全合成通过使用 TiCl 4促进的对称1,2,7,8-四酮的抗选择性羟醛环化作为关键反应分 7 步完成。


























 京公网安备 11010802027423号
京公网安备 11010802027423号