当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rapid construction of a polysubstituted “caged” oxa-bishomocubane framework from vinylidenecyclopropanes through a sequential dual catalysis of copper(I) and visible-light-induced photosensitization
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-05-26 , DOI: 10.1039/d2qo00508e Yan-Shun Zhang 1 , Yin Wei 2 , Min Shi 1, 2
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-05-26 , DOI: 10.1039/d2qo00508e Yan-Shun Zhang 1 , Yin Wei 2 , Min Shi 1, 2
Affiliation
|
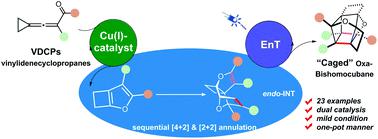
This study describes a sequential dual catalytic transformation involving copper(I)-catalyzed cyclization/isomerization/migration–dimerization and visible-light photo-induced intramolecular [2 + 2] cycloaddition of vinylidenecyclopropanes for the rapid construction of polysubstituted “caged” oxa-bishomocubane products. The reaction proceeds through an in situ generated cyclobutane-fused furan upon Cu(I) catalysis and its dimerization occurring via an intermolecular [4 + 2] cycloaddition event. These dimerized dihydrofuran derivatives could undergo an intramolecular [2 + 2] cycloaddition upon visible-light irradiation, affording polysubstituted “caged” oxa-bishomocubane derivatives in moderate to good yields. This newly developed synthetic protocol features a simple operation with readily available starting materials in good step-economy in the facile synthesis of a “caged” product. The plausible cascade catalytic reaction mechanism has been also proposed on the basis of control experiments and density functional theory (DFT) calculation.
中文翻译:

通过铜 (I) 的顺序双重催化和可见光诱导的光敏化,从亚乙烯基环丙烷中快速构建多取代的“笼状”氧杂-二叔戊烷骨架
本研究描述了一种顺序双催化转化,包括铜 ( I ) 催化的环化/异构化/迁移-二聚化和可见光光诱导的亚乙烯基环丙烷的分子内 [2 + 2] 环加成,用于快速构建多取代的“笼状”氧杂-二叔戊烷。产品。该反应通过在 Cu( I ) 催化下原位生成的环丁烷稠合呋喃进行,并通过以下途径发生二聚化分子间 [4 + 2] 环加成事件。这些二聚二氢呋喃衍生物可以在可见光照射下进行分子内 [2 + 2] 环加成反应,从而以中等至良好的产率提供多取代的“笼状”氧杂二氢呋喃衍生物。这种新开发的合成方案具有简单的操作和易于获得的起始材料,具有良好的步骤经济性,可轻松合成“笼式”产品。在控制实验和密度泛函理论 (DFT) 计算的基础上,还提出了似是而非的级联催化反应机理。
更新日期:2022-05-26
中文翻译:

通过铜 (I) 的顺序双重催化和可见光诱导的光敏化,从亚乙烯基环丙烷中快速构建多取代的“笼状”氧杂-二叔戊烷骨架
本研究描述了一种顺序双催化转化,包括铜 ( I ) 催化的环化/异构化/迁移-二聚化和可见光光诱导的亚乙烯基环丙烷的分子内 [2 + 2] 环加成,用于快速构建多取代的“笼状”氧杂-二叔戊烷。产品。该反应通过在 Cu( I ) 催化下原位生成的环丁烷稠合呋喃进行,并通过以下途径发生二聚化分子间 [4 + 2] 环加成事件。这些二聚二氢呋喃衍生物可以在可见光照射下进行分子内 [2 + 2] 环加成反应,从而以中等至良好的产率提供多取代的“笼状”氧杂二氢呋喃衍生物。这种新开发的合成方案具有简单的操作和易于获得的起始材料,具有良好的步骤经济性,可轻松合成“笼式”产品。在控制实验和密度泛函理论 (DFT) 计算的基础上,还提出了似是而非的级联催化反应机理。


























 京公网安备 11010802027423号
京公网安备 11010802027423号