Developmental Cell ( IF 11.8 ) Pub Date : 2022-05-24 , DOI: 10.1016/j.devcel.2022.05.004 Zunpeng Liu 1 , Qianzhao Ji 2 , Jie Ren 3 , Pengze Yan 2 , Zeming Wu 2 , Si Wang 4 , Liang Sun 5 , Zehua Wang 1 , Jiaming Li 6 , Guoqiang Sun 1 , Chuqian Liang 2 , Run Sun 1 , Xiaoyu Jiang 2 , Jianli Hu 7 , Yingjie Ding 7 , Qiaoran Wang 6 , Shijia Bi 1 , Gang Wei 8 , Gang Cao 9 , Guoguang Zhao 10 , Hongmei Wang 11 , Qi Zhou 11 , Juan Carlos Izpisua Belmonte 12 , Jing Qu 11 , Weiqi Zhang 3 , Guang-Hui Liu 13
|
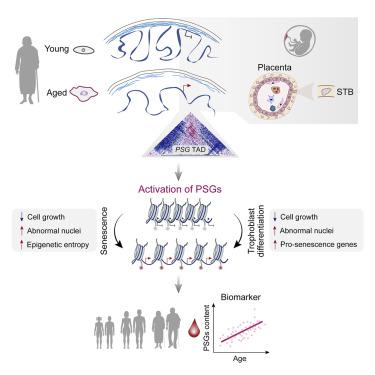
Nuclear deformation, a hallmark frequently observed in senescent cells, is presumed to be associated with the erosion of chromatin organization at the nuclear periphery. However, how such gradual changes in higher-order genome organization impinge on local epigenetic modifications to drive cellular mechanisms of aging has remained enigmatic. Here, through large-scale epigenomic analyses of isogenic young, senescent, and progeroid human mesenchymal progenitor cells (hMPCs), we delineate a hierarchy of integrated structural state changes that manifest as heterochromatin loss in repressive compartments, euchromatin weakening in active compartments, switching in interfacing topological compartments, and increasing epigenetic entropy. We found that the epigenetic de-repression unlocks the expression of pregnancy-specific beta-1 glycoprotein (PSG) genes that exacerbate hMPC aging and serve as potential aging biomarkers. Our analyses provide a rich resource for uncovering the principles of epigenomic landscape organization and its changes in cellular aging and for identifying aging drivers and intervention targets with a genome-topology-based mechanism.
中文翻译:

大规模染色质重组重新激活驱动细胞衰老的胎盘特异性基因
核变形是衰老细胞中经常观察到的标志,据推测与核外围染色质组织的侵蚀有关。然而,高阶基因组组织的这种逐渐变化如何影响局部表观遗传修饰以驱动细胞衰老机制仍然是个谜。在这里,通过对同基因年轻、衰老和早衰人类间充质祖细胞 (hMPC) 的大规模表观基因组分析,我们描绘了综合结构状态变化的层次结构,表现为抑制区室中的异染色质丢失、活跃区室中的常染色质减弱、切换连接拓扑隔间,并增加表观遗传熵。我们发现表观遗传去抑制释放了妊娠特异性 β-1 糖蛋白的表达。PSG ) 基因会加剧 hMPC 衰老并作为潜在的衰老生物标志物。我们的分析为揭示表观基因组景观组织的原理及其在细胞衰老中的变化以及通过基于基因组拓扑的机制确定衰老驱动因素和干预目标提供了丰富的资源。



























 京公网安备 11010802027423号
京公网安备 11010802027423号