Structure ( IF 5.7 ) Pub Date : 2022-05-23 , DOI: 10.1016/j.str.2022.05.002 Rob van der Kant 1 , Nikolaos Louros 1 , Joost Schymkowitz 1 , Frederic Rousseau 1
|
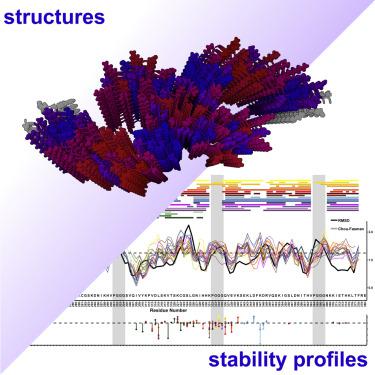
The increasing number of amyloid structures offers an opportunity to investigate the general principles determining amyloid stability and polymorphism. We find that amyloid stability is dominated by ∼30% of residues localized in segments that favor the cross-β conformation. These correspond to known aggregation-nucleating regions and constitute a stabilizing cross-β structural framework that is shared among polymorphs. Alternative packing of these segments with structurally frustrated regions within the protofilament results in conformationally different, but energetically similar, polymorphs. Differential analysis of distributions of interatomic distances in amyloid and globular structures revealed that unconventional residue contacts, such as identical charges in close proximity, are located in energetically frustrated segments of amyloids. These observations suggest that polymorphism results from a framework mechanism consisting of conserved stabilizing regions of high cross-β propensity. These are interspersed by structurally suboptimal regions that are potential sites of conformational plasticity and interaction with stabilizing cofactors such as (poly)ions.
中文翻译:

淀粉样蛋白原纤维结构的热力学分析揭示了淀粉样蛋白多晶型物稳定性的共同框架
越来越多的淀粉样蛋白结构为研究确定淀粉样蛋白稳定性和多态性的一般原理提供了机会。我们发现淀粉样蛋白的稳定性主要由位于有利于交叉β构象的片段中的约 30% 的残基决定。这些对应于已知的聚集成核区域并构成多晶型物之间共享的稳定的交叉β结构框架。这些片段与原丝内结构受挫区域的替代包装导致构象不同但能量相似的多晶型物。对淀粉样蛋白和球状结构中原子间距离分布的差异分析表明,非常规的残基接触,例如非常接近的相同电荷,位于淀粉样蛋白的能量受挫片段中。这些观察结果表明,多态性是由由高交叉β倾向的保守稳定区组成的框架机制引起的。这些散布在结构上不理想的区域,这些区域是构象可塑性的潜在位点,并与稳定的辅助因子(例如(多)离子)相互作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号