当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Asymmetric total syntheses of five pyrrole-type Stemona alkaloids
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-05-23 , DOI: 10.1039/d2qo00456a Xiaodong Wang 1 , Tao Shi 1 , Gaofeng Yin 1 , Yuqing Wang 1 , Zhao Li 1 , Zhen Wang 1, 2
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-05-23 , DOI: 10.1039/d2qo00456a Xiaodong Wang 1 , Tao Shi 1 , Gaofeng Yin 1 , Yuqing Wang 1 , Zhao Li 1 , Zhen Wang 1, 2
Affiliation
|
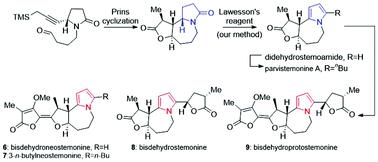
The asymmetric total syntheses of five pyrrole-type Stemona alkaloids and two stereoisomers were accomplished, among which 3-n-butylneostemonine and bisdehydroneostemonine were synthesized for the first time, and the NMR data of bisdehydroneostemonine were revised. Specifically, the 5/7 skeleton of stemoamide was established by employing Prins cyclization, and didehydrostemoamide was obtained from stemoamide using our method of Lawesson's reagent promoted pyrrole synthesis. Using didehydrostemoamide as the common intermediate, five pyrrole Stemona alkaloids were divergently synthesized. This research has enriched the transformation pattern of Stemona alkaloids.
中文翻译:

五种吡咯型百部生物碱的不对称全合成
完成了五种吡咯型百部生物碱和两种立体异构体的不对称全合成,其中首次合成了3-正丁基新百部碱和双脱氢新百部碱,并对双脱氢新百部碱的核磁共振数据进行了修正。具体而言,利用Prins环化建立了干酪酰胺的5/7骨架,并利用我们的劳森试剂促进吡咯合成的方法从干酪酰胺中获得了双脱氢百香酰胺。以双去氢百叶酰胺为共同中间体,分歧合成了五种吡咯百部生物碱。本研究丰富了百部生物碱的转化模式。
更新日期:2022-05-23
中文翻译:

五种吡咯型百部生物碱的不对称全合成
完成了五种吡咯型百部生物碱和两种立体异构体的不对称全合成,其中首次合成了3-正丁基新百部碱和双脱氢新百部碱,并对双脱氢新百部碱的核磁共振数据进行了修正。具体而言,利用Prins环化建立了干酪酰胺的5/7骨架,并利用我们的劳森试剂促进吡咯合成的方法从干酪酰胺中获得了双脱氢百香酰胺。以双去氢百叶酰胺为共同中间体,分歧合成了五种吡咯百部生物碱。本研究丰富了百部生物碱的转化模式。



























 京公网安备 11010802027423号
京公网安备 11010802027423号