Chem Catalysis Pub Date : 2022-05-20 , DOI: 10.1016/j.checat.2022.04.019 Alissa Bleem , Eugene Kuatsjah , Gerald N. Presley , Daniel J. Hinchen , Michael Zahn , David C. Garcia , William E. Michener , Gerhard König , Konstantinos Tornesakis , Marco N. Allemann , Richard J. Giannone , John E. McGeehan , Gregg T. Beckham , Joshua K. Michener
|
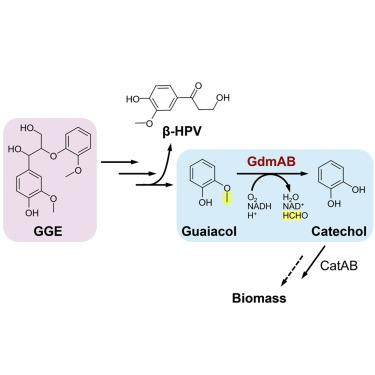
Aryl-O-demethylation is a common rate-limiting step in the catabolism of lignin-related compounds, including guaiacol. Here we used randomly barcoded transposon insertion sequencing (RB-TnSeq) in the bacterium Novosphingobium aromaticivorans to identify a Rieske-type guaiacol O-demethylase, GdmA. Similarity searches identified GdmA homologs in other bacteria, along with candidate reductase partners, denoted GdmB. GdmAB combinations were biochemically characterized for activity with several lignin-related substrates. Structural and sequence comparisons of vanillate- and guaiacol-specific O-demethylase active sites revealed conserved hallmarks of substrate specificity. GdmAB combinations were also evaluated in Pseudomonas putida KT2440, which does not natively utilize guaiacol. GdmAB from Cupriavidus necator N-1 demonstrated the highest rate of guaiacol turnover in vitro and in engineered P. putida strains and notably higher catalytic efficiency than a cytochrome P450 system (GcoAB) and the vanillate Rieske-type O-demethylase from P. putida (VanAB). The GdmAB O-demethylases described here expand the suite of options for microbial conversion of a model lignin-derived substrate.
中文翻译:

用于愈创木酚 O-去甲基化的 Rieske 非血红素铁单加氧酶的发现、表征和代谢工程
芳基-O-去甲基化是木质素相关化合物(包括愈创木酚)分解代谢中常见的限速步骤。在这里,我们在新鞘氨醇细菌中使用随机条形码转座子插入测序 (RB-TnSeq)来鉴定 Rieske 型愈创木酚O -去甲基化酶 GdmA。相似性搜索确定了其他细菌中的 GdmA 同源物,以及候选还原酶伙伴,表示为 GdmB。GdmAB 组合的生化特征是与几种木质素相关底物的活性。香草酸和愈创木酚特异性O-去甲基酶活性位点的结构和序列比较揭示了底物特异性的保守标志。GdmAB 组合也在Pseudomonas putida KT2440,本身不利用愈创木酚。来自Cupriavidus necator N-1 的 GdmAB在体外和工程化恶臭假单胞菌菌株中表现出最高的愈创木酚转换率,并且催化效率显着高于来自恶臭假单胞菌的细胞色素 P450 系统( GcoAB) 和香草酸盐 Rieske 型O-脱甲基酶。范AB)。此处描述的 GdmAB O-去甲基化酶扩展了用于微生物转化模型木质素衍生底物的选项套件。



























 京公网安备 11010802027423号
京公网安备 11010802027423号