当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rhodium(II)-Catalyzed Site-Selective Intramolecular Insertion of Aryldiazoacetates into Unactivated Primary C−H Bond: A Direct Route to 2-Unsubstituted Indanes
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2022-05-20 , DOI: 10.1002/adsc.202200115 Hisanori Nambu 1 , Ryoya Amano 1 , Takafumi Tamura 1 , Takayuki Yakura 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2022-05-20 , DOI: 10.1002/adsc.202200115 Hisanori Nambu 1 , Ryoya Amano 1 , Takafumi Tamura 1 , Takayuki Yakura 1
Affiliation
|
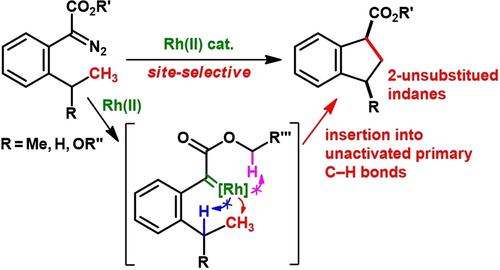
Dirhodium(II)-catalyzed intramolecular insertion of aryldiazoacetates into an unactivated primary C−H bond was described in this study. The insertion reaction of aryldiazoacetates with ortho-isopropyl or ortho-ethyl groups in the presence of a catalytic amount of dirhodium(II) tetrakis(triphenylacetate), Rh2(tpa)4, at room temperature proceeded site-selectively to afford 2-unsubstituted indane-1-carboxylates in 75%–96% yields. In the case of o-isopropyl-substituted aryldiazoacetates, cis-isomers were obtained as major products. Furthermore, a chemoselective C−H insertion reaction of aryldiazoacetate bearing 1-oxyethyl-substituent at the ortho position was achieved by using a bulky and electron-deficient pivaloyl group as the protecting group of a highly active oxygen atom. The present reaction provided a direct route to 2-unsubstituted indanes.
中文翻译:

铑 (II) 催化的芳基重氮乙酸酯分子内选择性插入未活化的伯 C-H 键:直接生成 2-未取代的茚满
本研究描述了二铑 (II) 催化的芳基重氮乙酸酯分子内插入未活化的初级 C-H 键。在催化量的四(三苯基乙酸)二铑(II),Rh 2 (tpa) 4存在下,重氮乙酸芳基酯与邻-异丙基或邻-乙基的插入反应在室温下进行位点选择性,得到2-未取代indane-1-carboxylates 的产率为 75%–96%。在邻异丙基取代的芳基重氮乙酸酯的情况下,获得了顺式异构体作为主要产物。此外,在邻位带有 1-氧乙基取代基的芳基重氮乙酸酯的化学选择性 C-H 插入反应位置是通过使用庞大且缺电子的新戊酰基作为高活性氧原子的保护基团来实现的。本反应提供了获得 2-未取代的茚满的直接途径。
更新日期:2022-05-20
中文翻译:

铑 (II) 催化的芳基重氮乙酸酯分子内选择性插入未活化的伯 C-H 键:直接生成 2-未取代的茚满
本研究描述了二铑 (II) 催化的芳基重氮乙酸酯分子内插入未活化的初级 C-H 键。在催化量的四(三苯基乙酸)二铑(II),Rh 2 (tpa) 4存在下,重氮乙酸芳基酯与邻-异丙基或邻-乙基的插入反应在室温下进行位点选择性,得到2-未取代indane-1-carboxylates 的产率为 75%–96%。在邻异丙基取代的芳基重氮乙酸酯的情况下,获得了顺式异构体作为主要产物。此外,在邻位带有 1-氧乙基取代基的芳基重氮乙酸酯的化学选择性 C-H 插入反应位置是通过使用庞大且缺电子的新戊酰基作为高活性氧原子的保护基团来实现的。本反应提供了获得 2-未取代的茚满的直接途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号