当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
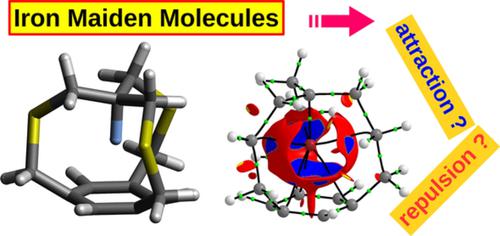
The physical nature of the ultrashort spike–ring interaction in iron maiden molecules
Journal of Computational Chemistry ( IF 3 ) Pub Date : 2022-05-20 , DOI: 10.1002/jcc.26879 Mirosław Jabłoński 1
Journal of Computational Chemistry ( IF 3 ) Pub Date : 2022-05-20 , DOI: 10.1002/jcc.26879 Mirosław Jabłoński 1
Affiliation
|
The so-called ‘iron maiden’ molecules belong to one of the most interesting subgroups of cyclophanes due to the presence of the ultrashort interaction between the CX apical bond and the benzene ring. This article presents an in-depth theoretical study of 16 ‘iron maiden’ molecules, in which X = H, F, Cl or Br and the side chains are of various lengths and types: CSC, CSCC, CCC, and CCCC. It is shown that the H → F → Cl → Br substitution leads to a significant expansion of the ‘iron maiden’ molecule. Shorter chains lead to more pronounced effects, while insertion of sulfur atoms into the side chains lowers them. Structural changes are associated with an increase in energetic destabilization of X. Moreover, unlike for H, in the case of X = halogen, the out → in isomerization is energetically disadvantageous. The ‘iron maiden’ molecules are characterized by the presence of only three X⋯CAr bond paths. Particularly noteworthy are unusually large (even up to 32) values of the X⋯CAr bond ellipticity, which results from flat electron density distribution. The X⋯π interaction in each of the investigated ‘iron maiden’ molecule turned out to be multi-center, stabilizing and almost purely covalent in nature as indicated by the definitely dominant percentage (94.8%–101.6%) of the exchange-correlation energy. The spatial hindrance within the ‘iron maiden’ molecules appears to be not so much due to the X⋯π repulsion, but due to unfavorable steric interactions between X and the CC side bonds. It is also confirmed that some CH⋯HC interactions in aliphatic chains can be very weakly stabilizing.
中文翻译:

铁少女分子中超短尖峰-环相互作用的物理性质
由于 C X 顶端键和苯环之间存在超短相互作用,所谓的“铁娘子”分子属于最有趣的环烷亚组之一。本文对 16 个“铁娘子”分子进行了深入的理论研究,其中 X = H、F、Cl 或 Br,侧链具有各种长度和类型:CSC、CSCC、CCC 和 CCCC。结果表明,H → F → Cl → Br 取代导致“铁娘子”分子的显着扩张。较短的链会导致更明显的效果,而将硫原子插入侧链会降低它们。结构变化与 X 的能量不稳定增加有关。此外,与 H 不同,在 X = 卤素的情况下,out → in异构化在能量上是不利的。“铁娘子”分子的特点是仅存在三个 X⋯C Ar键路径。特别值得注意的是 X⋯C Ar键椭圆率的值异常大(甚至高达 32),这是由平坦的电子密度分布引起的。每个被研究的“铁娘子”分子中的 X⋯ π相互作用结果证明是多中心的、稳定的并且在本质上几乎是纯共价的,如交换相关能量的绝对优势百分比 (94.8%–101.6%) 所示. “铁娘子”分子内的空间位阻似乎不是由于 X⋯ π排斥,而是由于 X 和 C 之间的不利空间相互作用C侧键。还证实脂肪链中的一些 CH⋯HC 相互作用可以非常弱地稳定。
更新日期:2022-05-20
中文翻译:

铁少女分子中超短尖峰-环相互作用的物理性质
由于 C X 顶端键和苯环之间存在超短相互作用,所谓的“铁娘子”分子属于最有趣的环烷亚组之一。本文对 16 个“铁娘子”分子进行了深入的理论研究,其中 X = H、F、Cl 或 Br,侧链具有各种长度和类型:CSC、CSCC、CCC 和 CCCC。结果表明,H → F → Cl → Br 取代导致“铁娘子”分子的显着扩张。较短的链会导致更明显的效果,而将硫原子插入侧链会降低它们。结构变化与 X 的能量不稳定增加有关。此外,与 H 不同,在 X = 卤素的情况下,out → in异构化在能量上是不利的。“铁娘子”分子的特点是仅存在三个 X⋯C Ar键路径。特别值得注意的是 X⋯C Ar键椭圆率的值异常大(甚至高达 32),这是由平坦的电子密度分布引起的。每个被研究的“铁娘子”分子中的 X⋯ π相互作用结果证明是多中心的、稳定的并且在本质上几乎是纯共价的,如交换相关能量的绝对优势百分比 (94.8%–101.6%) 所示. “铁娘子”分子内的空间位阻似乎不是由于 X⋯ π排斥,而是由于 X 和 C 之间的不利空间相互作用C侧键。还证实脂肪链中的一些 CH⋯HC 相互作用可以非常弱地稳定。


























 京公网安备 11010802027423号
京公网安备 11010802027423号