Metabolic Engineering ( IF 8.4 ) Pub Date : 2022-05-20 , DOI: 10.1016/j.ymben.2022.05.004 Mohammad S Radi 1 , Jesus E SalcedoSora 2 , Se Hyeuk Kim 1 , Suresh Sudarsan 1 , Anand V Sastry 3 , Douglas B Kell 4 , Markus J Herrgård 5 , Adam M Feist 6
|
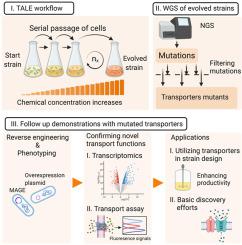
Membrane transport proteins are potential targets for medical and biotechnological applications. However, more than 30% of reported membrane transporter families are either poorly characterized or lack adequate functional annotation. Here, adaptive laboratory evolution was leveraged to identify membrane transporters for a set of four amino acids as well as specific mutations that modulate the activities of these transporters. Specifically, Escherichia coli was adaptively evolved under increasing concentrations of L–histidine, L–phenylalanine, L–threonine, and L–methionine separately with multiple replicate evolutions. Evolved populations and isolated clones displayed growth rates comparable to the unstressed ancestral strain at elevated concentrations (four-to six-fold increases) of the targeted amino acids. Whole genome sequencing of the evolved strains revealed a diverse number of key mutations, including SNPs, small deletions, and copy number variants targeting the transporters leuE for histidine, yddG for phenylalanine, yedA for methionine, and brnQ and rhtC for threonine. Reverse engineering of the mutations in the ancestral strain established mutation causality of the specific mutations for the tolerant phenotypes. The functional roles of yedA and brnQ in the transport of methionine and threonine, respectively, are novel assignments and their functional roles were validated using a flow cytometry cellular accumulation assay. To demonstrate how the identified transporters can be leveraged for production, an L–phenylalanine overproduction strain was shown to be a superior producer when the identified yddG exporter was overexpressed. Overall, the results revealed the striking efficiency of laboratory evolution to identify transporters and specific mutational mechanisms to modulate their activities, thereby demonstrating promising applicability in transporter discovery efforts and strain engineering.
中文翻译:

通过自适应实验室进化识别和调节膜转运蛋白
膜转运蛋白是医学和生物技术应用的潜在目标。然而,超过 30% 的已报道膜转运蛋白家族要么缺乏特征,要么缺乏足够的功能注释。在这里,利用适应性实验室进化来识别一组四种氨基酸的膜转运蛋白以及调节这些转运蛋白活性的特定突变。具体来说,大肠杆菌在 L-组氨酸、L-苯丙氨酸、L-苏氨酸和 L-蛋氨酸浓度增加的情况下自适应进化,并分别进行了多次重复进化。进化的种群和分离的克隆在目标氨基酸浓度升高(增加四到六倍)时显示出与未受胁迫的祖先菌株相当的生长速率。进化菌株的全基因组测序揭示了多种关键突变,包括 SNP、小缺失和针对组氨酸转运蛋白leuE、苯丙氨酸yddG、蛋氨酸 yedA 以及brnQ和rhtC的拷贝数变异为苏氨酸。祖先菌株中突变的逆向工程确定了耐受表型的特定突变的突变因果关系。yedA和brnQ分别在蛋氨酸和苏氨酸转运中的功能作用是新的分配,并且它们的功能作用通过流式细胞仪细胞积累测定得到验证。为了证明如何利用已鉴定的转运蛋白进行生产,当鉴定的yddG出口商被夸大了。总体而言,结果揭示了实验室进化在识别转运蛋白和调节其活性的特定突变机制方面的惊人效率,从而证明了在转运蛋白发现工作和菌株工程中的应用前景。


























 京公网安备 11010802027423号
京公网安备 11010802027423号