当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
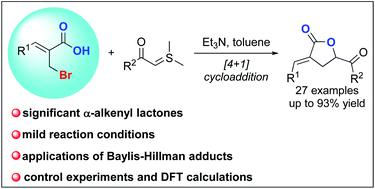
A formal [4 + 1] cycloaddition reaction of Baylis–Hillman bromides with sulfur ylides: facile access to α-alkenyl lactones
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-05-19 , DOI: 10.1039/d2qo00451h Ting-Bi Hua 1 , Yu-Hong Ma 1 , Xiao-Yu He 1 , Long Wang 1, 2 , Jia-Ying Yan 1, 2 , Qing-Qing Yang 1, 2
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-05-19 , DOI: 10.1039/d2qo00451h Ting-Bi Hua 1 , Yu-Hong Ma 1 , Xiao-Yu He 1 , Long Wang 1, 2 , Jia-Ying Yan 1, 2 , Qing-Qing Yang 1, 2
Affiliation
|
A formal [4 + 1] cycloaddition reaction of Baylis–Hillman adducts with sulfur ylides has been developed for the first time. This protocol features high functional group tolerance and provides facile access to biologically interesting α-alkenyl lactones with generally high yields. Meanwhile, the DFT calculation of the pathways has also been performed.
中文翻译:

Baylis-Hillman 溴化物与硫叶立德的正式 [4 + 1] 环加成反应:容易获得 α-烯基内酯
首次开发了 Baylis-Hillman 加合物与硫叶立德的正式 [4 + 1] 环加成反应。该协议具有高官能团耐受性,可轻松获得具有生物学意义的 α-烯基内酯,且产量普遍较高。同时,还进行了路径的 DFT 计算。
更新日期:2022-05-19
中文翻译:

Baylis-Hillman 溴化物与硫叶立德的正式 [4 + 1] 环加成反应:容易获得 α-烯基内酯
首次开发了 Baylis-Hillman 加合物与硫叶立德的正式 [4 + 1] 环加成反应。该协议具有高官能团耐受性,可轻松获得具有生物学意义的 α-烯基内酯,且产量普遍较高。同时,还进行了路径的 DFT 计算。


























 京公网安备 11010802027423号
京公网安备 11010802027423号