当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Facile Preparation of UFMylation Activity-Based Probes by Chemoselective Installation of Electrophiles at the C-Terminus of Recombinant UFM1
ACS Central Science ( IF 18.2 ) Pub Date : 2022-05-17 , DOI: 10.1021/acscentsci.2c00203 Kateryna A Tolmachova 1 , Jakob Farnung 1 , Jin Rui Liang 2 , Jacob E Corn 2 , Jeffrey W Bode 1
ACS Central Science ( IF 18.2 ) Pub Date : 2022-05-17 , DOI: 10.1021/acscentsci.2c00203 Kateryna A Tolmachova 1 , Jakob Farnung 1 , Jin Rui Liang 2 , Jacob E Corn 2 , Jeffrey W Bode 1
Affiliation
|
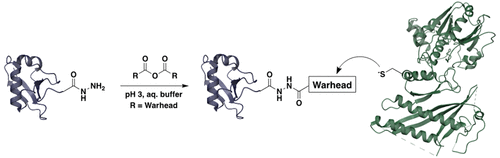
Aberrations in protein modification with ubiquitin-fold modifier (UFM1) are associated with a range of diseases, but the biological function and regulation of this post-translational modification, known as UFMylation, remain enigmatic. To provide activity-based probes for UFMylation, we have developed a new method for the installation of electrophilic warheads at the C-terminus of recombinant UFM1. A C-terminal UFM1 acyl hydrazide was readily produced by selective intein cleavage and chemoselectively acylated by a variety of carboxylic acid anhydrides at pH 3, without detriment to the folded protein or reactions at unprotected amino acid side chains. The resulting UFM1 activity-based probes show a range of tunable reactivity and high selectivity for proteins involved in UFMylation processes; structurally related E1s, E2s, and proteases associated with Ub or other Ubls were unreactive. The UFM1 probes were active both in cell lysates and in living cells. A previously inaccessible α-chloroacetyl probe was remarkably selective for covalent modification of the active-site cysteine of de-UFMylase UFSP2 in cellulo.
中文翻译:

通过在重组 UFM1 的 C 端化学选择性安装亲电试剂,轻松制备基于 UFMylation 活性的探针
泛素折叠修饰剂 (UFM1) 对蛋白质修饰的异常与一系列疾病有关,但这种翻译后修饰(称为 UFMylation)的生物学功能和调节仍然是个谜。为了为 UFMylation 提供基于活性的探针,我们开发了一种在重组 UFM1 的 C 末端安装亲电子弹头的新方法。C 端 UFM1 酰基酰肼很容易通过选择性内含肽裂解产生,并在 pH 3 下被各种羧酸酐化学选择性酰化,而不会损害折叠的蛋白质或未受保护的氨基酸侧链的反应。由此产生的基于 UFM1 活性的探针显示出一系列可调反应性和对参与 UFMylation 过程的蛋白质的高选择性;结构相关的 E1、E2、与 Ub 或其他 Ubl 相关的蛋白酶没有反应性。UFM1 探针在细胞裂解物和活细胞中均有活性。以前无法接近的 α-氯乙酰基探针对去 UFMylase UFSP2 的活性位点半胱氨酸的共价修饰具有显着的选择性在纤维素中。
更新日期:2022-05-17
中文翻译:

通过在重组 UFM1 的 C 端化学选择性安装亲电试剂,轻松制备基于 UFMylation 活性的探针
泛素折叠修饰剂 (UFM1) 对蛋白质修饰的异常与一系列疾病有关,但这种翻译后修饰(称为 UFMylation)的生物学功能和调节仍然是个谜。为了为 UFMylation 提供基于活性的探针,我们开发了一种在重组 UFM1 的 C 末端安装亲电子弹头的新方法。C 端 UFM1 酰基酰肼很容易通过选择性内含肽裂解产生,并在 pH 3 下被各种羧酸酐化学选择性酰化,而不会损害折叠的蛋白质或未受保护的氨基酸侧链的反应。由此产生的基于 UFM1 活性的探针显示出一系列可调反应性和对参与 UFMylation 过程的蛋白质的高选择性;结构相关的 E1、E2、与 Ub 或其他 Ubl 相关的蛋白酶没有反应性。UFM1 探针在细胞裂解物和活细胞中均有活性。以前无法接近的 α-氯乙酰基探针对去 UFMylase UFSP2 的活性位点半胱氨酸的共价修饰具有显着的选择性在纤维素中。

























 京公网安备 11010802027423号
京公网安备 11010802027423号