Structure ( IF 5.7 ) Pub Date : 2022-05-16 , DOI: 10.1016/j.str.2022.04.009 Artem N Bonchuk 1 , Konstantin M Boyko 2 , Alena Y Nikolaeva 3 , Anna D Burtseva 2 , Vladimir O Popov 4 , Pavel G Georgiev 5
|
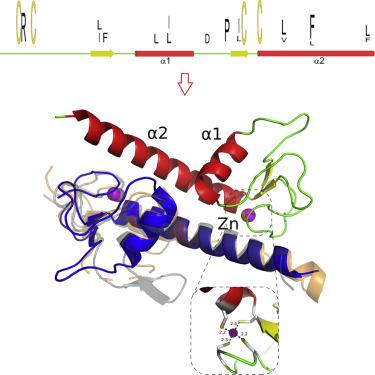
ZAD is a C4 zinc-coordinating domain often found at the N-terminus mostly of arthropodan transcription factors with multiple C2H2 zinc-finger domains involved in the regulation of chromosome architecture and promotor activity. ZADs predominantly form homodimers and have low primary sequence similarity. We obtained three crystal structures of the most phylogenetically distant Drosophila ZADs and structure of the only known ZAD-like domain from a mammalian protein (ZNF276). All ZAD structures demonstrate unity of the spatial fold as well as some unique structural features. The specific homodimerization of ZAD is primarily determined by the position and size of secondary structural elements and is further strengthened by a number of unique interactions between subunits. Structural comparison allowed for unraveling key sequence features underlying the similarity of the spatial fold. These features result in a broad variety of ZADs in Arthropod C2H2 proteins, allowing for the emergence of a wide range of highly specific homodimers.
中文翻译:

对序列相似性非常低的锌指相关域的高度相似空间组织的结构洞察
ZAD 是一个 C4 锌配位结构域,通常在 N 端发现,主要是节肢动物转录因子,具有多个 C2H2 锌指结构域,参与调节染色体结构和启动子活性。ZAD 主要形成同源二聚体并且具有低一级序列相似性。我们获得了系统发育最远的果蝇的三个晶体结构ZAD 和哺乳动物蛋白 (ZNF276) 中唯一已知的 ZAD 样结构域的结构。所有 ZAD 结构都表现出空间褶皱的统一性以及一些独特的结构特征。ZAD 的特异性同二聚化主要由二级结构元素的位置和大小决定,并通过亚基之间的许多独特相互作用进一步加强。结构比较允许解开空间折叠相似性背后的关键序列特征。这些特征导致节肢动物 C2H2 蛋白中的 ZAD 种类繁多,从而允许出现各种高度特异性的同源二聚体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号