Cell Host & Microbe ( IF 30.3 ) Pub Date : 2022-05-13 , DOI: 10.1016/j.chom.2022.04.013 Rebecca A Drummond 1 , Jigar V Desai 2 , Emily E Ricotta 2 , Muthulekha Swamydas 2 , Clay Deming 3 , Sean Conlan 3 , Mariam Quinones 4 , Veronika Matei-Rascu 5 , Lozan Sherif 5 , David Lecky 5 , Chyi-Chia R Lee 6 , Nathaniel M Green 2 , Nicholas Collins 7 , Adrian M Zelazny 8 , D Rebecca Prevots 2 , David Bending 5 , David Withers 5 , Yasmine Belkaid 9 , Julia A Segre 3 , Michail S Lionakis 2
|
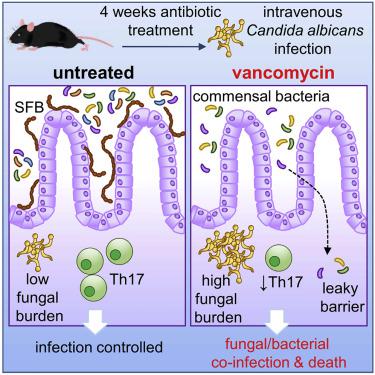
Antibiotics are a modifiable iatrogenic risk factor for the most common human nosocomial fungal infection, invasive candidiasis, yet the underlying mechanisms remain elusive. We found that antibiotics enhanced the susceptibility to murine invasive candidiasis due to impaired lymphocyte-dependent IL-17A- and GM-CSF-mediated antifungal immunity within the gut. This led to non-inflammatory bacterial escape and systemic bacterial co-infection, which could be ameliorated by IL-17A or GM-CSF immunotherapy. Vancomycin alone similarly enhanced the susceptibility to invasive fungal infection and systemic bacterial co-infection. Mechanistically, vancomycin reduced the frequency of gut Th17 cells associated with impaired proliferation and RORγt expression. Vancomycin’s effects on Th17 cells were indirect, manifesting only in vivo in the presence of dysbiosis. In humans, antibiotics were associated with an increased risk of invasive candidiasis and death after invasive candidiasis. Our work highlights the importance of antibiotic stewardship in protecting vulnerable patients from life-threatening infections and provides mechanistic insights into a controllable iatrogenic risk factor for invasive candidiasis.
中文翻译:

长期接触抗生素会导致淋巴细胞功能障碍和共生菌全身逃逸,从而增加全身真菌感染后的死亡率
抗生素是最常见的人类院内真菌感染、侵袭性念珠菌病的可改变的医源性危险因素,但其潜在机制仍然难以捉摸。我们发现,由于肠道内淋巴细胞依赖性 IL-17A 和 GM-CSF 介导的抗真菌免疫受损,抗生素增强了小鼠对侵袭性念珠菌病的易感性。这导致非炎症性细菌逃逸和全身性细菌合并感染,可以通过 IL-17A 或 GM-CSF 免疫疗法来改善。单独使用万古霉素同样会增加对侵袭性真菌感染和全身性细菌合并感染的易感性。从机制上讲,万古霉素降低了与增殖和 RORγt 表达受损相关的肠道 Th17 细胞的频率。万古霉素对 Th17 细胞的作用是间接的,仅在体内表现出来在存在菌群失调的情况下。在人类中,抗生素与侵袭性念珠菌病和侵袭性念珠菌病后死亡的风险增加有关。我们的工作强调了抗生素管理在保护弱势患者免受危及生命的感染方面的重要性,并为侵袭性念珠菌病的可控医源性危险因素提供了机制见解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号